| 1 Capricor Therapeutics, Inc. Nasdaq: CAPR December 3, 2025 HOPE-3 Phase 3 Study Topline Data Call |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Forward Looking Statements Statements in this presentation regarding the efficacy, safety, and intended utilization of Capricor’s product candidates; the initiation, conduct, size, timing and results of clinical trials; the pace of enrollment of clinical trials; plans regarding regulatory filings, future research and clinical trials; regulatory developments involving products, including future interactions with regulatory authorities and the ability to obtain regulatory approvals or otherwise bring products to market; manufacturing capabilities; dates for regulatory meetings; the potential that required regulatory inspections may be delayed or not be successful which would delay or prevent product approval; the ability to achieve product milestones and to receive milestone payments from commercial partners; and any other statements about Capricor’s management team’s future expectations, beliefs, goals, plans or prospects constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words “believes,” “plans,” “could,” “anticipates,” “expects,” “estimates,” “should,” “target,” “will,” “would” and similar expressions)should also be considered to be forward-looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward-looking statements. More information about these and other risks that may impact Capricor’s business is set forth in Capricor’s Annual Report on Form 10-K for the year ended December 31, 2024, as filed with the Securities and Exchange Commission on March 26, 2025, and in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2025, as filed with the Securities and Exchange Commission on November 10, 2025. All forward-looking statements in this presentation are based on information available to Capricor as of the date hereof, and Capricor assumes no obligation to update these forward-looking statements. Capricor has entered into an agreement for the exclusive commercialization and distribution of Deramiocel for Duchenne muscular dystrophy in the United States and Japan with Nippon Shinyaku Co., Ltd. (U.S. subsidiary: NS Pharma, Inc.),subject to regulatory approval. Deramiocel and the StealthX vaccine are investigational candidates and have not been approved for commercial use in any indication. 2 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Attendees Linda Marbán, Ph.D. Chief Executive Officer, Capricor Therapeutics AJ Bergmann, M.B.A. Chief Financial Officer, Capricor Therapeutics Michael Binks, M.D. Chief Medical Officer, Capricor Therapeutics Mark Awadalla Chief Development Officer, Capricor Therapeutics Kati Maharry, Ph.D., Senior Director of Biostatistics, Capricor Therapeutics Nathan Hogan, Ph.D., Director of Biostatistics, Capricor Therapeutics Craig McDonald, M.D. Professor and Chair of the Department of Physical Medicine and Rehabilitation and Director of the Neuromuscular Disease Clinics at the University of California, Davis. He is the national PI of the Capricor HOPE-3 Trial. Jonathan Soslow, M.D., MSCI Professor of Pediatrics and Director of Clinical Research, Pediatric Cardiology; Co-Director, Duchenne Multispecialty Clinic; Director of Pediatric Cardiac Magnetic Resonance, Vanderbilt University Medical Center 3 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Agenda Introduction Deramiocel Program Overview HOPE-3 Phase 3 Trial Overview HOPE-3 Patient Demographics HOPE-3 Safety Profile Overview HOPE-3 Efficacy Overview Conclusions and Next Steps Q&A 4 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside DMD: A Devastating Rare Disease High Unmet Needs Across the Entire Disease Trajectory Whole Muscle Tissue Dystrophin Muscle-Fiber Membrane Dystrophin is a structural protein located within the muscle fiber membrane Acts both as a cushion and glue Without dystrophin, muscles (cardiac and skeletal) are unable to function properly, suffer progressive damage and eventually die Much of the muscle injury that occurs in dystrophin-deficiency is attributable to secondary damage caused by inflammation 5 |
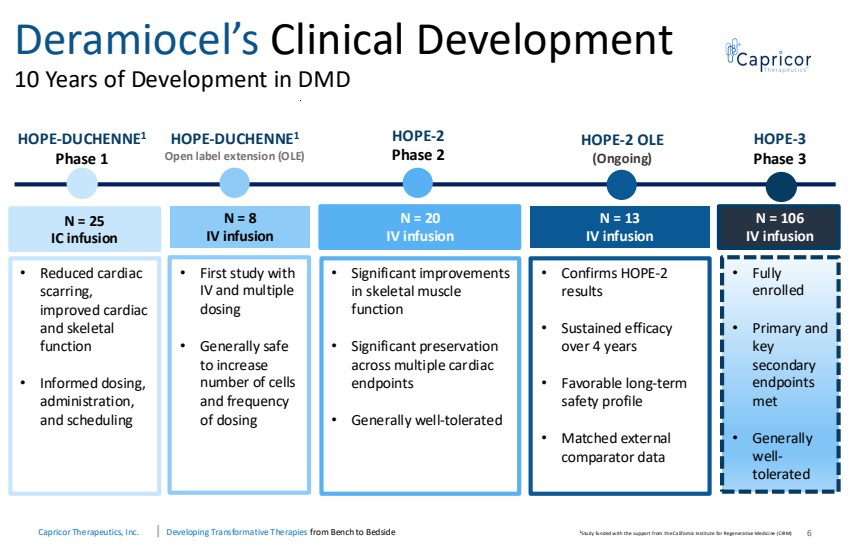
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel’s Clinical Development 10 Years of Development in DMD 6 HOPE-DUCHENNE1 Phase 1 HOPE-DUCHENNE1 Open label extension (OLE) HOPE-2 Phase 2 HOPE-2 OLE (Ongoing) HOPE-3 Phase 3 N = 25 IC infusion N = 8 IV infusion N = 20 IV infusion N = 13 IV infusion N = 106 IV infusion • Reduced cardiac scarring, improved cardiac and skeletal function • Informed dosing, administration, and scheduling • First study with IV and multiple dosing • Generally safe to increase number of cells and frequency of dosing • Significant improvements in skeletal muscle function • Significant preservation across multiple cardiac endpoints • Generally well-tolerated • Fully enrolled • Primary and key secondary endpoints met • Generally well-tolerated • Confirms HOPE-2 results • Sustained efficacy over 4 years • Favorable long-term safety profile • Matched external comparator data 1 S tudy funded with the support from the Ca lifornia Institute for Regenera tive Medici ne (CIRM) |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel’s Multi-Modal Mechanism Anti-fibrotic1 Anti-inflammatory Immunomodulatory1 1de Couto et al., 2015 CDCs Exosomes and growth factors 0 20 40 60 80 100 Control CDC cxcl8 0 20 40 60 80 100 120 Collagen CDC 0 2 4 6 8 M1 M2 MCDC M Φ Phenotype Toxic Protective 7 |
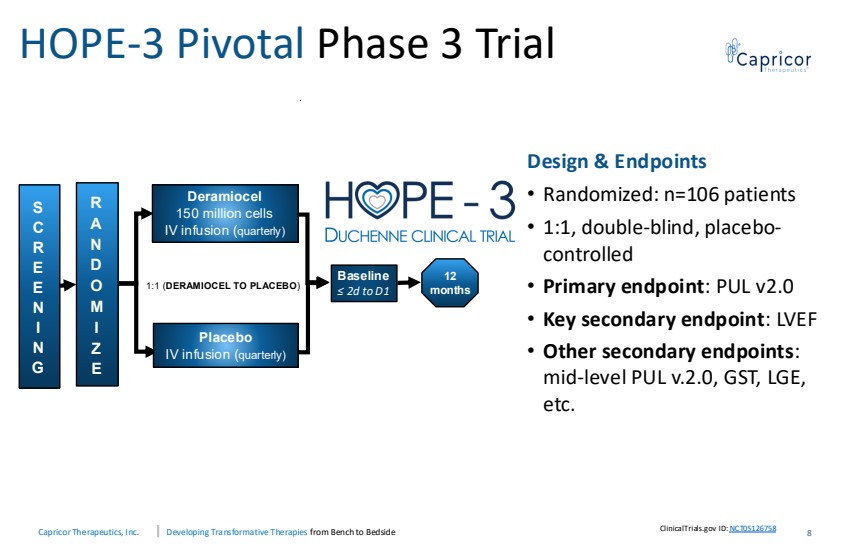
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE-3 Pivotal Phase 3 Trial S C R E E N I N G R A N D O M I Z E Deramiocel 150 million cells IV infusion (quarterly) 1:1 (DERAMIOCEL TO PLACEBO) 12 months Placebo IV infusion (quarterly) Baseline ≤ 2d to D1 Design & Endpoints • Randomized: n=106 patients • 1:1, double-blind, placebo-controlled • Primary endpoint: PUL v2.0 • Key secondary endpoint: LVEF • Other secondary endpoints: mid-level PUL v.2.0, GST, LGE, etc. 8 ClinicalTrials.gov ID:NCT05126758 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Approaching Loss of Ambulation Late-ambulatory patients with DMD and 10MWR > 10 sec 9 0 1 2 3 4 5 6 Performance of Upper Limb (PUL) Entry 2 to 6 (Target Population) 10 sec 10 MWR 100% lose ambulation within 24 months 10 sec 10 MWR indicates onset of upper extremity loss of function (Time to change from PUL entry 6 to PUL entry 5) McDonald CM, Signorovitch J, Mercuri E, et al. PLoS One. 2024 Jun 3;19(6):e0304099. |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Baseline Demographics Placebo (n=52) Deramiocel (n=54) Overall (n=106) Age (Years) n 52 54 106 Mean (SD) 14.6 (2.95) 15.4 (3.10) 15.0 (3.04) Median 14 15 15 Min, Max 10, 22 10, 22 10, 22 PUL 2.0 Entry Item Score 2,3 23 (44.2) 25 (46.3) 48 (45.3) 4,5,6 29 (55.8) 29 (53.7) 58 (54.7) Diagnosed Cardiomyopathy No 13 (25.0) 10 (18.5) 23 (21.7) Yes 39 (75.0) 44 (81.5) 83 (78.3) Baseline LVEF% n 46 45 91 Mean (SD) 59.303 (6.108) 55.345 (7.743) 57.346 (7.206) Median 59.309 55.892 57.532 Min, Max 47.395, 73.981 36.537, 71.112 36.537, 73.981 Ambulatory Status Non-Ambulatory 44 (84.6) 46 (85.2) 90 (84.9) Ambulatory 8 (15.4) 8 (14.8) 16 (15.1) HOPE-3 Demographics 10 |
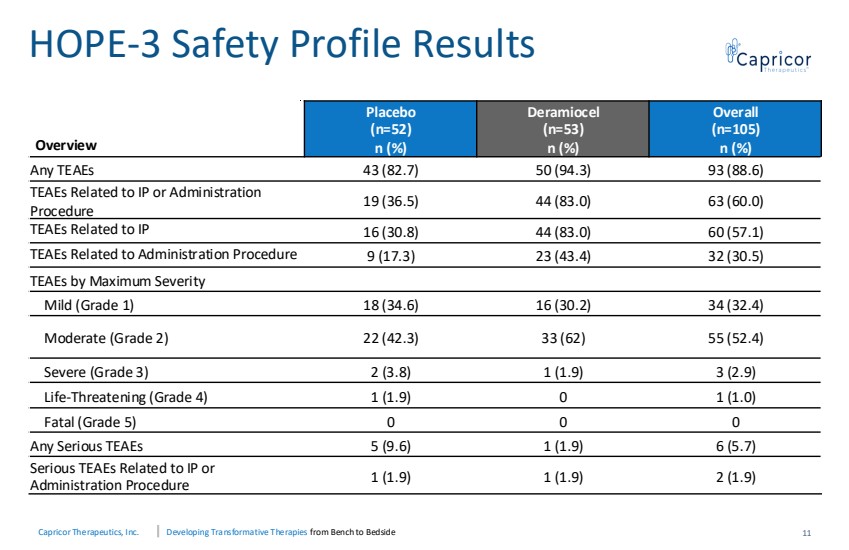
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Overview Placebo (n=52) n (%) Deramiocel (n=53) n (%) Overall (n=105) n (%) Any TEAEs 43 (82.7) 50 (94.3) 93 (88.6) TEAEs Related to IP or Administration Procedure 19 (36.5) 44 (83.0) 63 (60.0) TEAEs Related to IP 16 (30.8) 44 (83.0) 60 (57.1) TEAEs Related to Administration Procedure 9 (17.3) 23 (43.4) 32 (30.5) TEAEs by Maximum Severity Mild (Grade 1) 18 (34.6) 16 (30.2) 34 (32.4) Moderate (Grade 2) 22 (42.3) 33 (62) 55 (52.4) Severe (Grade 3) 2 (3.8) 1 (1.9) 3 (2.9) Life-Threatening (Grade 4) 1 (1.9) 0 1 (1.0) Fatal (Grade 5) 0 0 0 Any Serious TEAEs 5 (9.6) 1 (1.9) 6 (5.7) Serious TEAEs Related to IP or Administration Procedure 1 (1.9) 1 (1.9) 2 (1.9) HOPE-3 Safety Profile Results 11 |
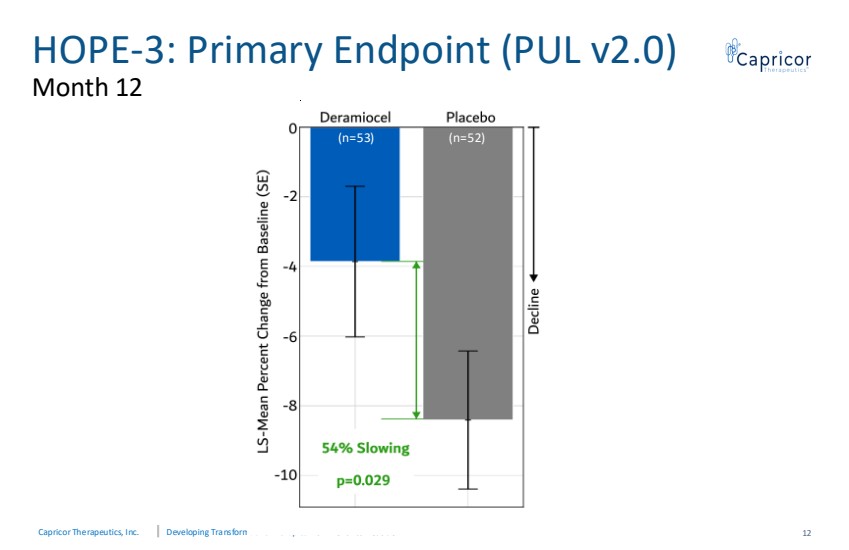
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE-3: Primary Endpoint (PUL v2.0) Month 12 12 (n=53) (n=52) |
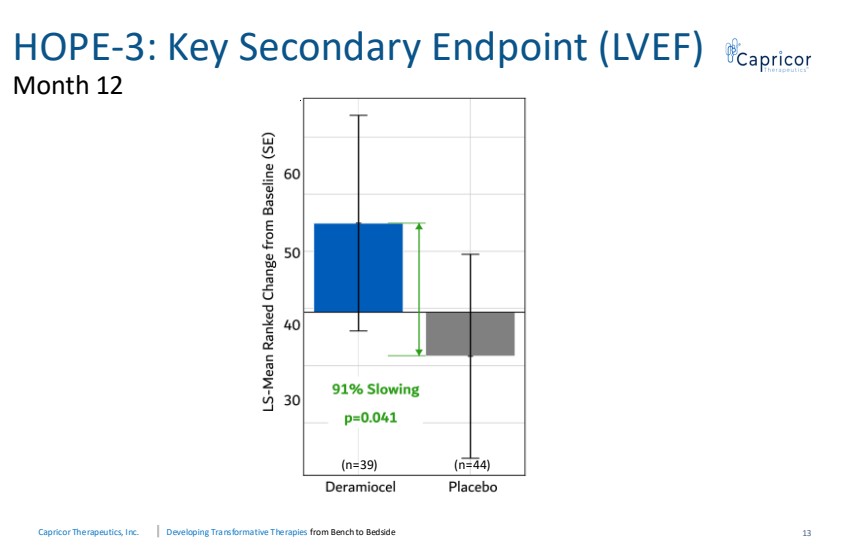
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE-3: Key Secondary Endpoint (LVEF) Month 12 13 (n=39) (n=44) |
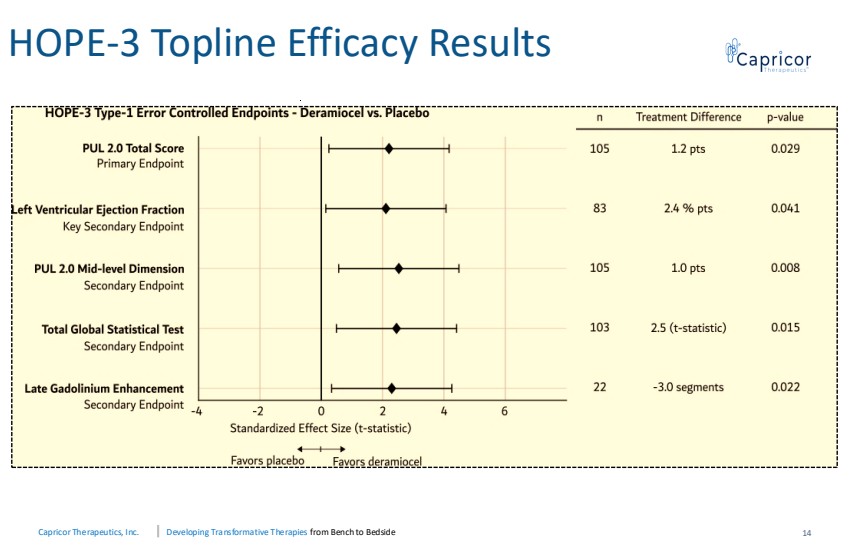
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside HOPE-3 Topline Efficacy Results 14 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Deramiocel has the Potential to Redefine the Standard of Care for Duchenne 15 Deramiocel Potential first-in-class therapy for DMD CARDIAC AND SKELETAL MUSCLE GENE THERAPIES EXON SKIPPING THERAPIES CORTICOSTEROIDS STANDARD CARDIAC MEDICATIONS Deramiocel can be used in combination with existing therapeutics |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Conclusions and Next Steps 16 • Pivotal Phase 3 study met the primary endpoint (PUL v2.0) and the key secondary cardiac endpoint (LVEF), both achieving statistical significance (p=0.03 and p=0.04) • Statistical significance was achieved in all type 1 error controlled secondary endpoints • Deramiocel is a potential first-in-class therapy designed to treat DMD skeletal and cardiomyopathy. • Deramiocel maintained a favorable safety and tolerability profile consistent with prior clinical experience • Plan to submit response to the Complete Response Letter incorporating HOPE-3 data, following prior alignment with FDA |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Acknowledgments All patients and their families who participated in the HOPE studies • Craig McDonald, M.D. (UC Davis Health), HOPE-3 National PI • Jonathan Soslow, M.D., MSCI, (Vanderbilt University Medical Center) • Chet Villa, M.D. (CCMC) • HOPE-3 investigators • All Duchenne advocacy organizations 17 |
| Capricor Therapeutics, Inc. Developing Transformative Therapies from Bench to Bedside Thank you Questions 18 |