Exhibit 10.37
*** Text Omitted and Filed Separately
Confidential Treatment Requested
Under 17 C.F.R. §§ 200.80(b)(4)
and 240.24b-2
COLLABORATION AGREEMENT AND LICENSE OPTION
This Collaboration Agreement and License Option (this “Agreement”), dated as of December 27, 2013 (the “Effective Date”), is made by and between Capricor, Inc., a Delaware corporation, having its principal place of business at 8840 Wilshire Boulevard, 2nd Floor, Beverly Hills, California 90211 (“Capricor”), and Janssen Biotech, Inc., a Pennsylvania corporation, and having an office at 800/850 Ridgeview Drive, Horsham, Pennsylvania 19044 (“JBI”). Capricor and JBI are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
WHEREAS, Capricor is a biotechnology company that conducts pharmaceutical research, development, and manufacturing;
WHEREAS, Capricor is developing certain products containing CDCs and CAP-1002 (each as defined below) for the treatment, diagnosis or prevention of cardiac diseases, disorders, and conditions and other therapeutic use, has access to intellectual property rights to certain CDCs and Cardiospheres (as defined below), and has developed extensive know-how relating to the manufacturing of such CDCs and Cardiospheres and data from pre-clinical and clinical studies of CDCs and Cardiospheres;
WHEREAS, JBI has existing pharmaceutical research, development, manufacturing, importing, exporting, commercialization, and selling capabilities;
WHEREAS, Capricor and JBI each desire to collaborate to develop CDC Cells and CDC Products (as defined below), in accordance with the terms and conditions set forth below.
NOW, THEREFORE, in consideration of the mutual promises contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound, the Parties hereto agree as follows:
1. DEFINITIONS.
The following terms and their correlatives have the following meanings:
1.1. “Affiliate” means any corporation or other entity which directly or indirectly controls, is controlled by or is under common control with a Party, for so long as such control exists. For the purposes of this Section 1.1 (“Affiliate”), “control” shall mean: (i) in the case of any corporate entity, direct or indirect ownership of more than fifty percent (50%) of the stock having the right to vote for the election of directors thereof or (ii) in the case of any non-corporate entity, direct or indirect ownership of more than fifty percent (50%) of the equity or income interest therein.
1.2. “Agreement” shall have the meaning set forth in the Preamble.
1.3. “ALLSTAR Dossier” means the ALLSTAR Trial Six Month Top Line Report and all raw data related thereto.
EXECUTION COPY
1.4. “ALLSTAR Trial” means the human clinical trial entitled Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration having the ClinicalTrials.gov identifier of NCT01458405, as may be amended.
1.5. “Business Day” means a day other than Saturday, Sunday, or bank or other public holiday in Beverly Hills, California.
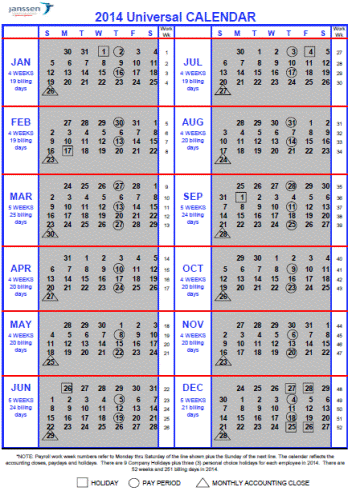
1.6. “Calendar Quarter” means a financial quarter based on the Johnson & Johnson Universal Financial Calendar for that year, a copy of which, for 2014 is attached hereto as Johnson & Johnson Universal Financial Calendar Schedule and which is used for JBI’s internal and external reporting purposes.
1.7. “Calendar Year” means the universal calendar that Johnson & Johnson uses as part of its financial reporting system, as provided to Capricor from time to time and as consistent with the 2014 universal calendar attached hereto as the Johnson & Johnson Universal Financial Calendar.
1.8. “CAP-1002” means, for the purposes of this Agreement, allogeneic cardiosphere derived Cells tested in the ALLSTAR trial.
1.9. “Capricor” shall have the meaning set forth in the Preamble.
1.10. “Capricor Indemnitees” shall have the meaning set forth in Section 11.1 (Indemnification by JBI).
1.11. “Cardiosphere(s)” means multicellular clusters containing allogeneic Cells developed from human cardiac tissue grown in culture.
1.12. “Cardiosphere Derived Cells” or “CDCs” mean Cell(s) that result from dissociation of Cardiospheres or subsequent culture of such Cells.
1.13. “CDC Cells” means CAP-1002 and allogeneic Cardiospheres and CDCs to which JBI may obtain an exclusive license pursuant the Exclusive License Agreement Term Sheet.
1.14. “CDC Product” means any pharmaceutical product in any dosage form containing CDC Cells.
1.15. “Cells” means a biological unit capable of division to produce further units, and consisting of a nucleus, other organelles and cytoplasm containing macromolecules including nucleic acids, proteins, polysaccharides, and lipids, bounded by a membrane.
1.16. “Claims” has the meaning set forth in Section 11.1 (Indemnification by JBI).
1.17. “CMC Committee” means the committee described in Section 5.1 (CMC Committee).
| CONFIDENTIAL | Page 2 |
EXECUTION COPY
1.18. “CMC Development Budget” shall have the meaning set forth in Section 2.1(h) (CMC Development Budget).
1.19. “CMC Development Plan” means the written plan for manufacturing CDC Cells and CDC Products in the Field by the Parties under FDA and EMA regulations an initial draft of which is attached as CMC Development Plan Schedule, attached hereto, and which may be amended from time to time in accordance with the terms of this Agreement.
1.20. “Commercially Reasonable Efforts” means, with respect to an objective, active and sustained efforts to achieve such objective in as prompt a manner as is reasonably practicable under the circumstances consistent with those efforts and resources that a party of similar size and resources in the pharmaceutical/biotechnology industry would normally use with respect to achieving such objective.
1.21. “Confidential Information” means the meaning set forth in Section 8.1 (Confidentiality; Exceptions).
1.22. “Control” means, with respect to any Information, Patent Right or other intellectual property right, the possession (whether by ownership or license) by a Party or its Affiliate of the conditional or unconditional ability to grant to the other Party access, ownership, a license or a sublicense as required herein to such Information, Patent Right, or other intellectual property right without violating the terms of any agreement or other arrangement with any Third Party in existence as of the Effective Date. In the case that the ability to grant is conditional (as with certain sublicenses), Control will require that the other Party be able to and agrees to satisfy such condition(s).
1.23. “Effective Date” has the meaning set forth in the Preamble.
1.24. “Exclusive License Agreement Term Sheet” means the term sheet attached to this Agreement in the Exclusive License Agreement Term Sheet Schedule.
1.25. “Disputed Amount(s)” means invoice amounts that are subject to a bona fide dispute raised by Capricor within thirty (30) days of Capricor’s receipt of such invoice.
1.26. “Development” or “Develop” means non-clinical and clinical drug development activities pertaining to a pharmaceutical product, including toxicology, pharmacology, test method development and stability testing, process and manufacturing development, formulation development, delivery system development, quality assurance and quality control development, statistical analysis, clinical studies (including pre- and post-approval studies), regulatory affairs, pharmacovigilance and regulatory approval and clinical study regulatory activities (including regulatory activities directed to obtaining pricing and reimbursement approvals).
1.27. “EMA” means the European Medicines Agency, and any successor agency thereto.
| CONFIDENTIAL | Page 3 |
EXECUTION COPY
1.28. “European Union” means the member states that are signatories to the Treaty of the European Union at the relevant time during the Term of this Agreement.
1.29. “Exploit” means to research, Develop, make, have made, use, import, export, commercialize, sell and offer for sale. Cognates of the word “Exploit” shall have correlative meanings.
1.30. “FDA” means the United States Food and Drug Administration or any successor agency thereto.
1.31. “Field” means the treatment, diagnosis or prevention of cardiac diseases, disorders, and conditions.
1.32. “Force Majeure” has the meaning set forth in Section 14.7(Force Majeure).
1.33. “FTE” means a full-time person, or more than one person working the equivalent of a full-time person, where “full-time” is considered one thousand seven hundred fifty (1750) hours per Calendar Year. No individual may record more than 1.0 FTE (i.e., no overtime paid), except in the case of documented, invoiced overtime paid to Third Party contractors for services directly related to CDC Product.
1.34. “FTE Payments” has the meaning set forth in Section 6.2(a) (FTE/OOP Payment).
1.35. “FTE Rate” means a rate of […***…] FTE per annum for one (1) FTE. FTE Rate shall be adjusted annually in proportion to the Consumer Price Index for all Urban Consumers for July of prior year to July of current year, as published by the U.S. Department of Labor, Bureau of Statistics (national CPI-U; Base Period: 1982-84=100; available at http://www.bls.gov/cpi/home.htm). Rate changes shall be effective as of the first day of the 1st Calendar Quarter of the Calendar Year.
1.36. “Indemnifying Party” has the meaning set forth in Article 11.
1.37. “Indemnified Party” has the meaning set forth in Article 11.
1.38. “Information” means all information not generally known to the public, including tangible and intangible techniques, technology, practices, trade secrets, inventions (whether patentable or not), methods, knowledge, know-how, conclusions, skill, experience, test data and results (including pharmacological, toxicological, manufacturing, and clinical test data and results), analytical and quality control data, results or descriptions, software and algorithms, including works of authorship and copyrights.
1.39. “Interest. Rate” means two percent (2%) plus the thirty (30) day U.S. Dollar LIBOR rate effective for the date that payment was due, as published by The Wall Street Journal, Eastern U.S. Edition, on the date such payment was due (or, if unavailable on such date, the first date thereafter on which such rate is available), or, if lower, the maximum rate permitted by Law.
*Confidential Treatment Requested
| CONFIDENTIAL | Page 4 |
EXECUTION COPY
1.40. “Invention(s)” has the meaning set forth in Section 7.1 (Inventions Ownership).
1.41. “Joint Steering Committee” or “JSC” means the committee described in Section 2.2 (Joint Steering Committee).
1.42. “Law” means, individually and collectively, any and all laws, ordinances, rules, directives, administrative circulars and regulations of any kind whatsoever of any Governmental Authority within the applicable jurisdiction.
1.43. “JBI” shall have the meaning set forth in the Preamble.
1.44. “JBI Know-How” means Information Controlled by JBI or its Affiliate that is necessary or useful for the Exploitation of a CDC Product.
1.45. “JBI Indemnitees” has the meaning set forth in Section 11.2(Indemnification by Capricor).
1.46. “Losses” has the meaning set forth in Section 11.1 (Indemnity by JBI).
1.47. “Materials” means any tangible chemical or biological material, including any libraries, DNA, RNA, clones, cells, and any expression product, progeny, derivative or other improvement thereto.
1.48. “Out- of- Pocket Costs” shall mean the amounts paid to Third Party vendors or contractors, for services, materials, equipment or supplies provided by them directly in the performance of activities under the CMC Development Plan, to the extent such services, materials, equipment or supplies apply directly to the CDC Product.
1.49. “OOP Payment” has the meaning set forth in Section 6.2(a) (FTE/OOP Payments).
1.50. “Option” means the right of JBI pursuant to this Agreement, to enter into with Capricor an Exclusive License Agreement pursuant to Section 4.3 (Option Grant).
1.51. “Option Period” has the meaning set forth in Section 4.3 (Option Exercise).
1.52. “Party” and “Parties” has the meaning set forth in the Preamble.
1.53. “Patent Right” means any and all (a) patents, (b) pending patent applications, including, all provisional applications, substitutions, continuations, continuations-in-part, divisions and renewals and all patents granted thereon, (c) all patents-of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms, including, supplementary protection certificates or the equivalent thereof, (d) inventor’s certificates, (e) any other form of government-issued right substantially similar to any of the foregoing, and (f) all U.S. and foreign counterparts of any of the foregoing.
| CONFIDENTIAL | Page 5 |
EXECUTION COPY
1.54. “Phase II Trial” or “Phase IIb Trial” means, with respect to the United States, any human clinical trial conducted in the specific patient population with the disease or condition of interest intended to be studied in a Phase III Trial for the purposes of preliminary assessment of safety and efficacy in the indication being studied, as described under 21 C.F.R. §312.21(b), and that, if the defined end-points are met, is sufficient to allow the Initiation of a Phase III Trial in the indication being studied, or, with respect to a jurisdiction other than the United States, an equivalent clinical study.
1.55. “Phase III Trial” means, with respect to the United States, any human clinical trial, that, if the defined end-points are met, is intended to be a pivotal trial for obtaining Regulatory Approval in the indication being studied or to otherwise establish safety and efficacy in patients with the indication being studied for purposes of filing for Regulatory Approval with the United States Food and Drug Administration (or its successor) as required under 21 C.F.R. §312.21(c), or, with respect to a jurisdiction other than the United States, an equivalent clinical study. In the event that a human clinical trial that would otherwise meet the definition of a Phase II Trial would, if the defined end-points are met, be sufficient to obtain Regulatory Approval in the indication being studied then, for purposes of this Agreement, such trial is considered a Phase III Trial.
1.56. “Prosecution and Maintenance” means, with respect to a Patent Right, the preparing, filing, and prosecuting of patent applications and maintenance of patents, as well as re-examinations, and reissues, with respect to such patents, together with the conduct of interferences and the defense of oppositions with respect to the particular patent application or patent; and “Prosecute and Maintain” shall have the correlative meaning.
1.57. “Regulatory Filing” means any filing with any Governmental Authority with respect to the development, manufacture, marketing, commercialization or reimbursement of a pharmaceutical product.
1.58. “Six Month Top Line Report” means a report generated by Capricor that will include the tables and listings set forth in the Six Month Top Line Report Schedule from the ALLSTAR Trial and may include such additional items agreed by the Parties.
1.59. “Term” means the period beginning on the Effective Date and ending upon the termination of this Agreement pursuant to Article 12 (Term and Termination).
1.60. “Third Party” means any entity other than a Party or an Affiliate of a Party.
1.61. “United States” or “U.S.” means the United States of America, including its territories and possessions, the District of Columbia and Puerto Rico.
| CONFIDENTIAL | Page 6 |
EXECUTION COPY
2. DEVELOPMENT PLAN
2.1 CMC Development Plan.
(a) Within forty-five (45) days of the Effective Date, the Parties shall amend the CMC Development Plan to add top line operational details (which includes a detailed budget of the amount that Janssen may invoice for and the financial commitments to be made by Capricor for the CMC work (such budget, the “CMC Development Budget”)). All manufacturing Development activities for CDC Cells and CDC Products contemplated by the Parties hereunder shall be set forth and carried out pursuant to the CMC Development Plan.
(b) Amendments. The CMC Development Plan may be amended from time to time by the CMC Committee in accordance with this Agreement and such amendments will be reflected in the CMC Development Plan. The CMC Development Plan is to be reviewed as necessary at each meeting of the CMC Committee and at any other time upon the reasonable request of either Party.
(c) Diligence. Each Party shall use Commercially Reasonable Efforts to perform (itself or through its Affiliates or by permitted subcontracting) its respective obligations under the CMC Development Plan, and shall reasonably cooperate with and provide reasonable support to the other Party in such other Party’s performance of its responsibilities under the CMC Development Plan. The Parties acknowledge and agree, however, that no outcome or success is or can be assured and that failure to achieve desired results do not in and of itself constitute a breach or default of any obligation in this Agreement.
(d) Permitted Subcontracting. Each Party may subcontract any of its activities to be performed under the CMC Development Plan to a Third Party or to an Affiliate of the Party, provided that any such Third Party or Affiliate shall have entered into a written Agreement with such Party that includes terms and conditions protecting and limiting use and disclosure of Confidential Information, Materials and Information of the other Party at least to the same extent as under this Agreement, and requiring such Third Party or Affiliate, as applicable, and its employees, contractors and agents to grant such Party Control in and to any Patent Rights, Information and Materials created, conceived or reduced to practice in connection with the performance of any such subcontracted activities. Each Party shall remain responsible and liable for the performance by its Affiliates and subcontractors of its obligations hereunder, and shall cause its Affiliates and subcontractors to comply with the provisions of this Agreement, including, causing such third parties to make any and all assignments of ownership of intellectual property rights generated in carrying out a Party’s obligation in accordance with the terms of this Agreement.
(e) Records. Each Party shall maintain, or cause to be maintained, records of its activities under the CMC Development Plan in sufficient detail and in good scientific manner appropriate for scientific, patent and regulatory purposes, which shall properly reflect all work included in the CMC Development Plan consistent with its internal procedures and policies.
| CONFIDENTIAL | Page 7 |
(f) Reports. Each Party shall furnish to the CMC Committee a written report, within forty-five (45) days after the end of each Calendar Quarter, that (i) describes such Party’s progress under the CMC Development Plan during the relevant Calendar Quarter and (ii) includes a summary of the results and data generated by such Party under the CMC Development Plan during the relevant Calendar Quarter, in each case to the extent reasonably necessary to support and advance the CMC Development Plan. For clarity, the written report required in this subsection is in addition to any other reporting requirements under this Agreement.
(g) Materials. Each Party may furnish to the other Party, as reasonably required, samples of Materials. The Party receiving any Materials shall not distribute or otherwise allow the release of Materials to any Third Party, except for subcontracting, in each case as permitted hereunder. All Materials delivered to the receiving Party are provided “AS IS”, shall be used in compliance with all Laws, and shall be used with prudence and appropriate caution in any experimental work because not all of their characteristics may be known. In regard to the transfer of any Material between the Parties, unless specifically stated otherwise by the transferor as a condition to a voluntary transfer, such transfer of Material will also be a transfer of ownership to the transferee of the physical sample being transferred. Such transfer will not exhaust intellectual property rights attached to such Material. All voluntary transfers of Material under this Agreement may be subject to a Material Transfer Agreement executed by the Parties on mutually agreeable terms.
(h) CMC Development Budget. JBI and Capricor shall each expend the FTEs, internal costs and out-of-pocket expenses necessary for such Party to perform the activities allocated to it in accordance with the CMC Development Plan. In the conduct of the CMC Development Plan, each of JBI and Capricor may, at its sole option and own expense (unless otherwise agreed by the Parties), expend in excess of the expenses budgeted for performance of its activities for any phase of the CMC Development Plan. Unless otherwise agreed in writing by the Parties, Janssen may invoice Capricor under the CMC Development Budget not more than […***…] during Term of the Agreement.
2.2 Joint Steering Committee. Within thirty (30) days of the Effective Date, Capricor and JBI will assemble a JSC. Initially, the JSC will be composed of at least two, but no more than four, representatives of each Party, with an equal number appointed by each of Capricor and JBI. Each Party will provide a list of its representatives to the other Party within thirty (30) days after the Effective Date. Each Party will promptly notify the other Party in writing of any change in its appointed representatives. Each Party may invite employees and consultants to attend meetings of the JSC, subject to their agreement, who are bound to obligations of confidentiality, non-use, and assignment of inventions similar to those of that Party’s members of the JSC.
(a) Meetings. The JSC will hold its first meeting within thirty (30) days of the Effective Date. While in existence, the JSC will meet on a quarterly basis by audio or video teleconference and, at a minimum, twice each year in person (which in-person meeting will be held on an alternating basis between Beverly Hills, California and site of JBI or any of its Affiliates, such to be agreed by the Parties). Each Party will bear its own costs relating to any
*Confidential Treatment Requested
| CONFIDENTIAL | Page 8 |
JSC meeting. Meetings of the JSC are effective only if at least one representative of each Party is present at the meeting or participating by teleconference. The Parties will endeavor to schedule meetings of the JSC at least two (2) months in advance.
(b) Responsibilities. The duties of the JSC will include, but not be limited to, reviewing the progress of and amendments to the ALLSTAR Trial and reviewing Capricor’s progress to generating the ALLSTAR Dossier. In addition, the JSC will discuss any other work ongoing at Capricor in the Field as well as the design and planning for Phase IIb and III clinical trials.
3. ALLSTAR TRIAL.
(a) Within thirty (30) days of the Effective Date, Capricor shall provide JBI with the current clinical protocol for the ALLSTAR trial.
(b) Amendments. Any material amendments proposed by Capricor to the ALLSTAR clinical protocol shall be presented to the JSC prior to being implemented, if reasonably practicable. For clarity, Capricor’s ability to amend the ALLSTAR clinical protocol shall not alter Capricor’s obligations relative to the ALLSTAR Dossier.
(c) Diligence. Capricor shall use Commercially Reasonable Efforts to complete dosing, data analysis, and generate the ALLSTAR Dossier.
(d) Reports. Capricor shall furnish to the JSC a written report, within forty-five (45) days after the end of each Calendar Quarter that describes Capricor’s progress in the ALLSTAR Trial during the relevant Calendar Quarter.
(e) Regulatory Filings. Capricor shall file, in its own name, all Regulatory Filings for the ALLSTAR Trial and any other clinical studies performed during the Term.
4. GRANT OF LICENSE AND EXCLUSIVE OPTION
4.1 Capricor hereby grants to JBI a limited and non-exclusive license under intellectual property Controlled by Capricor solely to the extent needed for JBI to perform its obligations under the CMC Development Plan. No other right or license is granted by Capricor to JBI under this Agreement.
4.2 JBI hereby grants to Capricor a limited non-exclusive license under intellectual property Controlled by JBI solely to the extent needed for Capricor to perform its obligations under the CMC Development Plan. No other right or license is granted by JBI to Capricor under this Agreement.
4.3 Option Grant. Capricor grants to JBI an exclusive right pursuant to this Agreement to enter into an Exclusive License Agreement on terms which shall include (a) the financial terms, the definitions of Licensed Cells and Licensed Products and the License Grants specifically set forth in the Exclusive License Agreement Term Sheet; (b) other terms and conditions substantially the same as those in the Exclusive License Agreement Term Sheet; and (c) additional other customary terms and conditions to be negotiated and agreed by the Parties (the
| CONFIDENTIAL | Page 9 |
“Option”). From the Effective Date until the period ending sixty (60) days after delivery of the ALLSTAR Dossier by Capricor to JBI (the “Option Period”), JBI shall have the right to notify Capricor of its desire to commence negotiations for the Exclusive License Agreement. Upon receipt of such notice by Capricor, both Parties shall commence good faith negotiations to conclude and execute an Exclusive License Agreement prior to the end of the Option Period according to the terms of the Exclusive License Agreement Term Sheet. During the Option Period, Capricor agrees not to negotiate nor solicit interest from any Third Party for any rights to CDC Cells and CDC Products.
4.4 Upon delivery of the ALLSTAR Dossier, JBI shall promptly notify Capricor of any asserted deficiencies and Capricor shall respond to such request as promptly as reasonably practicable. Janssen shall immediately notify Capricor that the ALLSTAR Dossier is free of deficiencies. In any event, should Janssen not respond to Capricor within five (5) Business Days of JBI’s receipt of the ALLSTAR Dossier, the ALLSTAR Dossier shall be deemed to be delivered without deficiencies and the sixty (60) day period set forth in Section 4.3 shall start on the sixth business day of Janssen’s silence with respect to the ALLSTAR Dossier. Additionally, JBI may request that the ALLSTAR Dossier be supplemented with reasonable additional relevant and material data and other information, and if such a request is made, Capricor shall respond to such request as promptly as reasonably practicable.
4.5 If an Exclusive License Agreement is not executed within sixty (60) days from JBI’s receipt of the ALLSTAR Dossier, this Agreement will expire pursuant to Section 12.1 (Term).
4.6 Upon expiration of this Agreement by the failure of the Parties to mutually execute an Exclusive License Agreement prior to the end of the Option Period:
(a) JBI grants to Capricor a non-exclusive perpetual license to publish, disclose and use the Information of JBI that was utilized in the production of the clinical trial materials manufactured pursuant to the CMC Development Plan for any purpose; and
(b) JBI grants to Capricor an irrevocable, fully paid-up, nonexclusive license under patents Controlled by JBI utilized in the production of the clinical trial materials manufactured pursuant to the CMC Development Plan to make, use and sell CDC Products.
4.7 Prior to the expiration of the Option Period, Capricor shall provide JBI with an opportunity to review and comment on any amendment to any license listed in the Capricor Third Party License Schedule.
| 5. | GOVERNANCE. |
5.1. CMC Committee.
(a) CMC Committee. The Parties shall establish a CMC Committee, comprised of at least two (2) representatives of Capricor and at least two (2) representatives of JBI. As of the Effective Date, the representatives shall be […***…] for JBI (the “JBI CMC Members”) and […***…] for Capricor (the “Capricor CMC Members”). The number of Capricor CMC Members
*Confidential Treatment Requested
| CONFIDENTIAL | Page 10 |
shall be equal to the number of JBI CMC Members. Each Party may replace its representatives to the CMC Committee at any time upon written notice to the other Party. Each Party may invite non-voting employees and consultants to attend meetings of the CMC Committee, subject to their agreement, who are bound to obligations of confidentiality, non-use, and assignment of inventions similar to those of that Party’s members of the CMC Committee.
(b) CMC Committee Chair. The CMC Committee shall be chaired by a Capricor CMC Member (the “CMC Committee Chair”). As of the Effective Date the CMC Committee Chair shall be […***…]. The responsibilities of the CMC Committee Chair include:
(i) providing written notification to each Party at least thirty (30) days in advance of each CMC Committee meeting;
(ii) collecting and organizing agenda items for each CMC Committee meeting;
(iii) preparing the meeting agenda and circulating it for review and approval by the CMC Committee Members and preparing written minutes of such meeting within fifteen (15) Business Days after such meeting
(iv) preparing the written minutes of each CMC Committee meeting and circulating such minutes for review and approval by the Parties, identifying action items to be carried out by the Parties.
(c) Meetings. The CMC Committee shall hold its first meeting within thirty (30) days of the Effective Date. While in existence, the CMC Committee shall meet on a quarterly basis by audio or video teleconference and, at a minimum, twice each year in person (which in-person meeting shall be held on an alternating basis between Beverly Hills, California and site of JBI or any of its Affiliates, such to be agreed by the Parties). Each Party shall bear its own costs relating to any CMC Committee meeting. Meetings of the CMC Committee are effective only if at least one representative of each Party is present at the meeting or participating by teleconference. Each Party shall be responsible for all of its own expenses of participating in the committee meetings. The Parties shall endeavor to schedule meetings of the CMC Committee at least two (2) months in advance. The CMC Committee Chair shall prepare the meeting agenda and shall circulate for review and approval by the CMC Committee Members and prepare written minutes of such meeting within fifteen (15) Business Days after such meeting. In the course of the review of the agenda by the CMC Committee members, each Party shall have the right to add items to the agenda. The Parties shall agree on the minutes of each meeting promptly.
(d) Responsibilities. Following the initiation of the Development Program under the CMC Development Plan, the CMC Committee shall oversee and supervise the overall performance of the CMC Development Plan and within such scope shall: (i) discuss and review the progress of the CMC Development Plan, (ii) review proposed material amendments to the CMC Development Plan; (iii) review the efforts of the Parties under the CMC
*Confidential Treatment Requested
| CONFIDENTIAL | Page 11 |
Development Plan; (iv) assign activities under the CMC Development Plan consistent with the obligations of the Parties hereunder, and (v) to attempt to resolve any disputes on an informal basis.
(e) Decision-making. Regardless of the number of Capricor CMC Committee members or JBI CMC Committee members, each Party shall have one (1) vote, and the CMC Committee shall attempt to make decisions by consensus. In the event that a decision cannot be resolved by consensus, the chairperson shall make a final decision, provided, however, that the CMC Committee shall not make any decision that is inconsistent with the terms and conditions of this Agreement nor that would unilaterally impose (i) a financial obligation on the other Party beyond the scope of the agreed CMC Development Budget, or (ii) a technical obligation on the other Party that is beyond its current technical capability or capacity.
(f) Limits on CMC Committee Authority. Each Party shall retain the rights, powers and discretion granted to it under this Agreement and no such rights, powers, or discretion shall be delegated to or vested in the CMC Committee unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing. The CMC Committee shall not have the power to amend, modify or waive compliance with this Agreement (other than as expressly permitted hereunder).
| 6. | PAYMENTS. |
6.1. Development Fee. JBI shall pay to Capricor, within eight (8) Business Days subsequent to the Effective Date, a one-time payment of Twelve Million Five hundred thousand U.S. Dollars ($12,500,000), which is non-refundable and non-creditable and not subject to set-off.
6.2. Research Funding.
(a) FTE/OOP Payments. Capricor shall reimburse JBI for expenditures incurred in its performance under the CMC Development Plan including FTE costs actually incurred at the FTE Rate (such reimbursement, the “FTE Payments”) and Out of Pocket Costs (the “OOPs Payments”), in each case up to the maximum permitted under the CMC Development Budget for a given Calendar Quarter (such maximum referred to as “FTE/OOP Maximum Payment”). At the end of each Calendar Quarter, JBI shall provide Capricor with an invoice for the funded FTEs expended and reimbursable out of pocket expenses incurred during such Calendar Quarter up to the FTE/OOP Maximum Payment for that Calendar Quarter and Capricor shall reimburse all such amounts no later than forty-five (45) days after receipt of such invoice, excluding Disputed Amounts. The amount of a Disputed Amount may be withheld from payment of the specific invoice to which it relates. Disputed Amounts that Capricor subsequently agrees in writing to pay or that are required to be paid pursuant to a dispute resolution shall be paid within thirty (30) days from the date of such agreement or resolution. Any payments or portions thereof due hereunder that are not paid when due, excluding Disputed Amounts, will bear interest at the Interest Rate, calculated on the number of
| CONFIDENTIAL | Page 12 |
days such payment is delinquent. The payment of interest will in no way limit any other remedies available to JBI. Should an invoice not be paid in full, (excluding Disputed Amounts) for at least six (6) months, JBI shall have no obligation to carry out its assigned activities under the CMC Development Plan until such payment has been received by JBI. The Parties recognize that, on a Calendar Quarter by Calendar Quarter basis, the actual FTE/OOP Payment due may change based on actual work conducted, but the annual budget amount set forth in the CMC Development Plan shall not change unless mutually agreed to by the Parties. With regards to reimbursement for Out of Pocket Expenses, JBI shall provide documentation supporting such expenses, such as invoices or pro forma invoices from Third Party vendors. With regards to reimbursement for funded FTEs, JBI shall provide documentation supporting the usage of such FTEs, such as an FTE time report break down by function. At Capricor’s request (to be made no more than twice per Calendar Year), JBI shall provide Capricor with reasonable additional information to support FTE Payments, including documentation, reasonably requested by Capricor in support of invoiced FTE utilization.
(b) Records and Audits. JBI shall keep adequate books and records of accounting for all expenses incurred in carrying out the activities that were reimbursed using the FTE/OOP Payments and ensuring JBI’s compliance hereunder. For the three (3) years following the end of the Calendar Year to which each pertains, such books and records of accounting will be kept at each of their principal place of business and no more than once per Calendar Year will be open for inspection during normal business hours upon at least forty five (45) days’ prior written notice by an independent certified accountant selected by Capricor at Capricor’s expense, and which is reasonably acceptable to JBI, for inspecting expenditure under the FTE/OOP Payments made by Capricor under this Agreement. Such accountant shall have executed and delivered to JBI, a customary confidentiality agreement as reasonably requested by JBI. The results of such inspection, if any, will be shared by the accountant with Capricor and JBI at either of JBI’s or Capricor’s request, and are binding on both JBI and Capricor. Any overbillings, by Capricor’s choice, are to be paid either by being credited on the following Calendar Quarter’s invoice or reimbursed to Capricor via check within forty-five (45) days of notification of the results of such inspection. Any underpayments are to be included in the following Calendar Quarter’s invoice or paid separately consistent with the means in which JBI pays Capricor. Capricor shall pay for any such inspections, except that in the event there is a downward adjustment in billed expenses shown by such inspection of more than five percent (5%) of the amount billed over the period audited, JBI shall reimburse Capricor for any reasonable out-of-pocket costs of such accountant or related to such inspection. No Calendar Year will be subject to audit under this Section more than once.
(c) Manner of Payment. All payments to be made by a Party to another Party hereunder shall be by wire transfer to the relevant bank account detailed below or such other bank account as a Party (as applicable) may designate in writing from time to time during the Term.
| CONFIDENTIAL | Page 13 |
To Capricor:
Account name: […***…]
To JBI:
Bank name: […***…]
(d) Late Payments. Except as otherwise specifically stated herein, if any payment due to be made by either Party hereunder is not made when due, such late payment shall bear interest at the Interest Rate, calculated on the number of days such payment is delinquent.
| 7. | Intellectual Property |
7.1. Inventions Ownership. Subject to the provisions of Article 4 and this Article 7, Capricor shall own all right, title and interest in and to all Patent Rights that that are conceived, and/or reduced to practice pursuant to the CMC Development Plan solely or jointly by the employees, agents or subcontractors of each Party that are necessary for the Exploitation of CDC Cells and CDC Products (“Arising Patent Rights”). JBI and its Affiliates shall execute, and shall have its employees and subcontractors and those of its Affiliates execute all documents necessary to transfer all right, title and interest in and to any such inventions to Capricor.
7.2. Exploitation of Inventions. Subject to the terms and conditions of this Agreement, including the licenses granted herein, Capricor may Exploit (including sublicensing) any Arising Patent Right pursuant to Section 6.2 without accounting to or obtaining consent from JBI.
7.3. Joint Development Agreement. This Agreement is a joint research agreement in accordance with 35 U.S.C. §103(c)(3) to Develop and Commercialize CDC Products. No Party is required by this reference to have any Patent Right to take advantage of or become
*Confidential Treatment Requested
| CONFIDENTIAL | Page 14 |
subject to such §103(c)(3) except in accordance with the provisions of Article 7 regarding Prosecution and Maintenance of such Patent Right.
7.4. Prosecution and Maintenance. Capricor shall be responsible for the Prosecution and Maintenance and enforcement of Arising Patent Rights at its sole discretion and expense, unless otherwise agreed.
7.5. Defense and Settlement of Third Party Claims. If the Exploitation of any CDC Product is alleged by a Third Party to infringe a Third Party’s Patent Right or other intellectual property right, the Party becoming aware of such allegation shall promptly notify the other Party. The Party that is alleged to infringe the Third Party’s Patent Right or intellectual property right shall have the right to take such action as it deems appropriate in response to such allegation, and shall be solely responsible for all damages, costs and expenses in connection therewith, subject to the provisions of Article 9 (Representations, Warranties, and Covenants).
7.6. Capricor Third Party License. Notwithstanding any provision of this Article 7, if any provision conflicts with a Capricor Third Party License, the provision of the Capricor Third Party License controls.
7.7. Employee Agreements. Prior to beginning work relating to any aspect of the subject matter of this Agreement and/or being given access to Confidential Information of the other Party, each appropriate employee, consultant and/or agent of Capricor and JBI will have signed or will be bound to a commercially reasonable non-disclosure and/or invention assignment agreement. Each Party will be responsible for any compensation or payment to its employees, contractors or agents in connection with the invention of any Patent Right.
7.8. Cooperation. Each Party shall reasonably cooperate with the other Party in the Prosecution And Maintenance of the Arising Patent Rights pursuant to this Agreement. Such cooperation includes promptly executing all documents, or requiring inventors, subcontractors, employees, former employees (to the extent reasonably available) and consultants and agents to execute all documents, as reasonable and appropriate so as to enable the Prosecution And Maintenance or enforcement of any such Arising Patent Rights in any country.
| 8. | Confidentiality. |
8.1. Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing or required as a condition of sublicense, the Parties agree that, during the Term and for five (5) years thereafter, but in no case before July 10, 2022, the receiving Party will keep confidential and will not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any Information furnished to it by the other Party pursuant to this Agreement (collectively, “Confidential Information”). Notwithstanding the foregoing, Confidential Information will not include any information to the extent that it can be established by written documentation by the receiving Party that such information:
| CONFIDENTIAL | Page 15 |
(a) is obtained or was already known by the receiving Party or its Affiliates as a result of disclosure from a Third Party that the receiving Party neither knew nor should have known was under an obligation of confidentiality to the disclosing Party with respect to such information;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party through no act or omission of the receiving Party or its Affiliates in breach of this Agreement;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party or its Affiliates in breach of this Agreement; or
(d) was independently discovered or developed by the receiving Party or its Affiliates (without reference to or use of Confidential Information of the disclosing Party) as demonstrated by the receiving Party’s documented evidence prepared contemporaneously with such independent Development or other equally competent evidence.
8.2. Authorized Disclosure. Except as expressly provided otherwise in this Agreement, each Party may use and disclose Confidential Information of the other Party solely as follows: (a) under appropriate confidentiality provisions substantially equivalent to those in this Agreement: (i) in connection with the performance of its obligations or as reasonably necessary or useful in the exercise of its rights under this Agreement, and (ii) to the extent it believes such disclosure is reasonably necessary in conducting the activities contemplated under this Agreement; (b) to the extent such disclosure is to a Governmental Authority as reasonably necessary in filing or prosecuting patent, and trademark applications in accordance with this Agreement, prosecuting or defending litigation in accordance with this Agreement, complying with applicable governmental regulations with respect to performance under this Agreement, filing Regulatory Filings, obtaining regulatory approval or fulfilling post-approval regulatory obligations for an CDC Product, or otherwise required by Law, provided, however, that if a Party is required by Law or the rules of any securities exchange or automated quotation system to make any such disclosure of the other Party’s Confidential Information it will, except where impracticable for necessary disclosures (for example, in the event of medical emergency), give reasonable advance notice to the other Party of such disclosure requirement and, in the case of each of the foregoing, will use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed; (c) to advisors (including lawyers and accountants) on a need to know basis, in each case under appropriate confidentiality provisions or professional standards of confidentiality substantially equivalent to those of this Agreement; or (d) to the extent mutually agreed to by the Parties.
8.3. Confidential Treatment of Terms and Conditions. Neither Party shall disclose the terms and conditions of this Agreement except as may be required by Law or as necessary to effect terms of this Agreement, including the Option Right pursuant to 4.3 (Option Exercise). Notwithstanding the foregoing, with respect to complying with the disclosure requirements of any Governmental Authority in connection with any required filing of this Agreement, the Parties will consult with one another concerning which terms of this
| CONFIDENTIAL | Page 16 |
Agreement will be requested to be redacted in any public disclosure of the Agreement, and in any event each Party will seek reasonable confidential treatment for any public disclosure by any such Governmental Authority. Notwithstanding the foregoing, the Parties will agree upon and permit Capricor to release a press release to announce the execution of this Agreement, which is attached hereto as the Press Release Schedule for use in responding to inquiries about the Agreement; thereafter, JBI and Capricor may each disclose to Third Parties the information contained in such press release without the need for further approval by the other, provided that such information is still accurate. Each Party will additionally have the right to issue additional press releases in regards to this Agreement and/or the CDC Products only with the prior written approval of the other Party or as required to comply with any Law or by the rules of any stock exchange or automated quotation system (in the case of such required disclosure, by providing ten (10) Business Days’ notice to the other Party and reasonably considering comments provided by such other Party within three (3) Business Days after such notice). Any subsequent press release will contain the same accuracy and truthfulness as the original press release. An acceptable copy of this Agreement may be filed with the Securities and Exchange Commission, The New York Stock Exchange and/or the NASDAQ National Market as required by applicable law or regulation. In connection with such filing, the Parties will endeavor to obtain confidential treatment of economic and trade secret information.
8.4. Attorney-Client Privilege. Neither Party is waiving, nor will be deemed to have waived or diminished, any of its attorney work product protections, attorney-client privileges or similar protections and privileges as a result of disclosing information pursuant to this Agreement, or any of its Confidential Information (including Confidential Information related to pending or threatened litigation) to the receiving Party, regardless of whether the disclosing Party has asserted, or is or may be entitled to assert, such privileges and protections. The Parties: (a) share a common legal and commercial interest in such disclosure that is subject to such privileges and protections; (b) may become joint defendants in proceedings to which the information covered by such protections and privileges relates; (c) intend that such privileges and protections remain intact should either Party become subject to any actual or threatened proceeding to which the disclosing Party’s Confidential Information covered by such protections and privileges relates; and (d) intend that after the Effective Date both the receiving Party and the disclosing Party will have the right to assert such protections and privileges.
| 9. | Representations, Warranties and Covenants |
9.1. Mutual Representations and Warranties. In addition to the representations and warranties made by a Party elsewhere in this Agreement, each Party hereby represents and warrants to the other Party that:
(a) As of the Effective Date, it is duly organized and validly existing under the Laws of its jurisdiction of incorporation and it has full corporate power and authority and has taken all corporate action necessary to enter into and perform this Agreement;
(b) As of the Effective Date, this Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms; the execution, delivery
| CONFIDENTIAL | Page 17 |
and performance of the Agreement by such Party does not conflict with any agreement, instrument or understanding, oral or written, by which it is bound, nor to its knowledge as of the Effective Date violate any Law; and the person or persons executing this Agreement on such Party’s behalf have been duly authorized to do so by all requisite corporate action;
(c) As of the Effective Date, it has sufficient legal right and/or beneficial title or ownership of its respective Intellectual Property to grant the licenses to the other Party as purported to be granted pursuant to this Agreement.
9.2. Capricor Representations, Warranties and Covenants. In addition to the representations and warranties made by Capricor above and elsewhere in this agreement, Capricor hereby represents, warrants, and covenants to JBI that:
(a) As of the Effective Date, it has, or will have during the Term of this Agreement, the full right, power and authority to grant to JBI the licenses hereunder granted in this Agreement.
(b) As of the Effective Date, to the actual knowledge of Capricor or its Affiliates, there is no suit or legal proceeding pending or threatened in writing with respect to Capricor Intellectual Property, and
(c) As of the Effective Date, Capricor has not entered, and during the Term, will not knowingly enter, into any written agreement with a Third Party that conflicts with the rights granted to JBI hereunder or Capricor’s ability to fully perform its obligations hereunder.
9.3. JBI Representations, Warranties and Covenants In addition to the representations and warranties made by JBI above and elsewhere in this agreement, JBI hereby represents, warrants, and covenants to Capricor that:
(a) As of the Effective Date, it has, or will have during the Term of this Agreement, the full right, power and authority to grant to Capricor the licenses hereunder granted in this Agreement.
(b) As of the Effective Date, to the actual knowledge of JBI or its Affiliates, there is no suit or legal proceeding pending or threatened in writing with respect to JBI Intellectual Property, and
(c) As of the Effective Date, JBI has not entered, and during the Term, will not knowingly enter, into any written agreement with a Third Party that conflicts with the rights granted to Capricor hereunder or JBI’s ability to fully perform its obligations hereunder.
9.4. Disclaimer of Warranties. EXCEPT AS OTHERWISE SET FORTH IN ARTICLE 9 OF THIS AGREEMENT, JBI AND CAPRICOR EXPRESSLY DISCLAIM ANY AND ALL REPRESENTATIONS AND WARRANTIES, EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, WITH RESPECT TO THE INTELLECTUAL PROPERTY LICENSED INTHIS AGREEMENT, OR ANY OTHER SUBJECT MATTER
| CONFIDENTIAL | Page 18 |
RELATING TO THIS AGREEMENT, INCLUDING ANY WARRANTY OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, VALIDITY OR NONINFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS.
| 10. | Limitations of Liability; Insurance |
10.1. Limitations of Liability. IN NO EVENT WILL EITHER PARTY BE LIABLE TO THE OTHER PARTY FOR ANY INDIRECT, SPECIAL, INCIDENTAL, EXEMPLARY, MULTIPLE, CONSEQUENTIAL, OR PUNITIVE DAMAGES OF ANY KIND ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT, OR FOR ANY LOSS OR INJURY TO A PARTY'S PROFITS OR GOODWILL, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY (WHETHER IN CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT LIABILITY OR OTHERWISE), EVEN IF SUCH PARTY WAS ADVISED OR OTHERWISE AWARE OF THE LIKELIHOOD OF SUCH DAMAGES, EXCEPT WITH RESPECT TO CONSEQUENTIAL DAMAGES (WHICH IN NO EVENT WILL INCLUDE ANY PUNITIVE DAMAGES) AWARDED TO A PARTY THAT THE NON-BREACHING PARTY DEMOSTRATES RESULTED FROM A BREACH OF SECTION 8.1 (CONFIDENTIALITY; EXCEPTIONS), OR SECTION 8.2 (AUTHORIZED DISCLOSURE). NOTHING IN THIS SECTION 10.1 (LIMITATIONS OF LIABILITY) IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF EITHER PARTY UNDER ARTICLE 11 (INDEMNIFICATION) WITH RESPECT TO ANY DAMAGES PAID BY THE OTHER PARTY TO A THIRD PARTY IN CONNECTION WITH A THIRD PARTY CLAIM.
10.2. Insurance. During the Term and for […***…] thereafter, each Party shall obtain and maintain (i) commercial general liability insurance covering its obligations and activities hereunder and (ii) products liability insurance including coverage for CDC Products undergoing clinical trials with financially secure insurance carriers in a form and at levels as customary for a company of its size in the pharmaceutical or biotechnology industry (or reasonable self-insurance sufficient to provide materially the same level and type of protection).
| 11. | Indemnification. |
11.1. Indemnification by JBI. JBI will defend, indemnify, and hold harmless Capricor, its Affiliates and their respective directors, officers, employees, and agents (collectively, “Capricor Indemnitees”), at JBI’s cost and expense, from and against any and all liabilities, losses, costs, damages, fees or expenses (including reasonable legal expenses and attorneys’ fees incurred by any Capricor Indemnitees until such time as JBI has acknowledged and assumed its indemnification obligation hereunder with respect to a claim) (collectively, “Losses”) arising out of any claim, action, lawsuit, or other proceeding (collectively, “Claims”) brought against any Capricor Indemnitee by a Third Party to the extent such Losses result from (i) any negligence or willful misconduct on the part of JBI, its Affiliates, subcontractors, or sublicensees in performing its obligations under this Agreement, and (ii) a material breach by JBI of this Agreement, including the failure of JBI’s representations or warranties in Section 9.1 (Mutual Representations and Warranties) to be true in any material
*Confidential Treatment Requested
| CONFIDENTIAL | Page 19 |
respect, or (iii) any actual or alleged infringement or misappropriation by JBI or any of its Affiliates, subcontractors or sublicensees of any Patent Rights or other intellectual property rights in performing its obligations under this Agreement; provided, however, that JBI shall not be obligated to indemnify Capricor Indemnitees for any Losses to the extent such Losses arise as a result of (A) the material breach of any representation, warranty or covenant made by Capricor under this Agreement or (B) any negligence or willful misconduct on the part of any Capricor Indemnitee or (C) any action or omission undertaken at the direction or request of any Capricor Indemnitee.
11.2. Indemnification by Capricor. Capricor will defend , indemnify, and hold harmless JBI, its Affiliates and their respective directors, officers, employees, and agents (collectively, “JBI Indemnitees”), at JBI’s cost and expense, from and against any and all liabilities, losses, costs, damages, fees or expenses (including reasonable legal expenses and attorneys’ fees incurred by any JBI Indemnitees until such time as Capricor has acknowledged and assumed its indemnification obligation hereunder with respect to a claim) (collectively, “Losses”) arising out of any claim, action, lawsuit, or other proceeding (collectively, “Claims”) brought against any JBI Indemnitee by a Third Party to the extent such Losses result from (i) any negligence or willful misconduct on the part of Capricor, its Affiliates, subcontractors, or sublicensees in performing its obligations under this Agreement, and (ii) a material breach by Capricor of this Agreement, including the failure of Capricor’s representations or warranties in Section 9.1 (Mutual Representations and Warranties) to be true in any material respect, or (iii) any actual or alleged infringement or misappropriation by Capricor or any of its Affiliates, subcontractors or sublicensees of any Patent Rights or other intellectual property rights in performing its obligations under this Agreement; provided, however, that Capricor shall not be obligated to indemnify JBI Indemnitees for any Losses to the extent such Losses arise as a result of (A) the material breach of any representation, warranty or covenant made by JBI under this Agreement or (B) any negligence or willful misconduct on the part of any JBI Indemnitee or (C) any action or omission undertaken at the direction or request of any JBI Indemnitee.
11.3. Claim for Indemnification. Whenever any Claim or Loss arises for which a JBI Indemnitee or a Capricor Indemnitee (the “Indemnified Party”) may seek indemnification under this Article 11 (Indemnification), the Indemnified Party will promptly notify the other Party (the “Indemnifying Party”) of the Claim or Loss and, when known, the facts constituting the basis for the Claim or Loss; provided, however, that the failure by an Indemnified Party to give such notice or to otherwise meet its obligations under this Section 11.3 (Claim for Indemnification) will not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that the Indemnifying Party is actually prejudiced as a result of such failure. The Indemnifying Party will have exclusive control of the defense and settlement of all Claims for which it is responsible for indemnification and will assume the defense thereof at its own expense promptly upon notice of such Claim or Loss. The Indemnified Party will not settle or compromise any Claim by a Third Party for which it is entitled to indemnification without the prior written consent of the Indemnifying Party, unless the Indemnifying Party is in breach of its obligation to defend hereunder. In no event will the Indemnifying Party settle any Claim without the prior written consent of the Indemnified Party if such settlement does not include a complete release from liability on such
*Confidential Treatment Requested
| CONFIDENTIAL | Page 20 |
Claim or if such settlement would involve undertaking an obligation other than the payment of money, would bind or impair the Indemnified Party, or includes any admission of wrongdoing or that any intellectual property or proprietary right of the Indemnified Party is invalid or unenforceable. The Indemnified Party will reasonably cooperate with the Indemnifying Party at the Indemnifying Party’s expense and will make available to the Indemnifying Party reasonably requested information under the control of the Indemnified Party, which information will be subject to Article 8 (Confidentiality and Publications). The Indemnifying Party will permit the Indemnified Party to participate in (but not to control) the Third Party Claim through counsel of its choosing (to the extent it has the ability to do so). Notwithstanding any other provision of this subsection, if an Indemnified Party withholds his or her consent to a bona fide settlement offer, where but for such action, the Indemnifying Party could have settled such Claim, the Indemnifying Party will be required to indemnify the Indemnified Party only up to a maximum of the bona fide settlement offer for which the Indemnifying Party could have settled such Claim.
| 12. | TERM AND TERMINATION. |
12.1. Term. This Agreement shall commence as of the Effective Date and, unless sooner terminated in accordance with the terms hereof or by mutual written consent, shall expire upon the later of (i) the mutual execution of an Exclusive License Agreement pursuant to JBI’s exercising its Option or (ii) the expiration of the Option Period without the Exclusive License Agreement being executed (the “Term”).
12.2. Termination. This Agreement may be terminated as follows:
(a) Bankruptcy/Insolvency. Either Party may terminate this Agreement upon written notice or upon the filing or institution of a bankruptcy, reorganization, liquidation or receivership proceedings, or upon than assignment of a substantial portion of the assets for the benefit of creditors by the other party; provided however, that in the case of any involuntary bankruptcy or other proceeding such right to terminate shall only become effective if the party consents to the involuntary bankruptcy or other proceedings or such proceeding is not dismissed within ninety (90) days after the filing thereof.
(b) Section 365(n) Rights. All rights and licenses granted under or pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of rights to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. Each Party agrees that the other Party, as a licensee of intellectual property under this Agreement, shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. The Parties further agree that, in the event of a rejection of this Agreement by either Party (for purposes of this Section (b) (Section 365(n) Rights), the “licensor”) in any bankruptcy proceeding by or against the licensor under the U.S. Bankruptcy Code, (a) the other Party (for purposes of this Section 12.2 (Section 365(n) Rights), the “licensee”) will be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in the licensee’s possession, will be promptly delivered to it upon the licensee’s written request therefor, and (b) the licensor will not interfere with the licensee’s
| CONFIDENTIAL | Page 21 |
rights to intellectual property and all embodiments of intellectual property, and will assist and not interfere with the licensee in obtaining intellectual property and all embodiments of intellectual property from another entity. The term “embodiments” of intellectual property includes all tangible, intangible, electronic or other embodiments of rights and licenses hereunder, including all compounds and products embodying intellectual property, CDC Products, regulatory filings and related rights, and technology.
12.3. Safety Concern. If Capricor determines that the use of a CDC Product is likely to cause a clinical effect that is detrimental to the safety of patients, Capricor shall immediately notify JBI of such determination. The Parties shall meet and confer as soon as practicable regarding the facts leading to such determination and possible mitigation of such outcome.
12.4. Termination for Uncured Material Breach. In its sole discretion, either Party may terminate the Agreement upon the uncured material breach of the other Party.
12.5. Effect of Termination or Expiration. Upon the effective date of termination or Expiration of the Term, except as otherwise expressly provided herein, all rights and obligations of each Party hereunder shall cease, including all rights, licenses and sublicenses granted by a Party.
(i) No later than ten (10) days after the Termination Date, unless another period is agreed to in writing by the Parties, JBI may provide an invoice in respect of the final FTE/OOPs Payment due and payable for such work performed by JBI. JBI shall provide Capricor with a final invoice for the funded FTEs expended and reimbursable out of pocket and Capricor shall reimburse all such amounts no later than forty-five (45) Calendar Days after receipt of such invoice.
(ii) If this Agreement is Terminated by mutual consent, expires or is terminated for material breach by Capricor, pursuant to this Section 12:
(A) if requested by Capricor, JBI will, at Capricor’s cost, transfer CMC Information not already in Capricor’s possession to Capricor or a CMO designated by Capricor as necessary or useful for Capricor or such CMO to manufacture CDC Cells and CDC Products; and
(B) JBI shall grant to Capricor an exclusive, worldwide license to JBI’s right, title, and interest in and to JBI Know-How to Exploit CDC Cells and CDC Product in the Field.
12.6. Accrued Rights. Expiration or termination of this Agreement (or any provision hereof) for any reason shall be without prejudice to any right that shall have accrued to the benefit of a Party prior to such expiration or termination, including damages arising from any
| CONFIDENTIAL | Page 22 |
breach under this Agreement. Expiration or termination of this Agreement shall not relieve a Party from any obligation that is expressly indicated to survive such expiration or termination.
12.7. Survival. The following provisions shall survive termination or expiration of this Agreement: Section 1 (Definitions), Section 4.6, Section 6.2 (Research Funding) Article 7 (Intellectual Property), Article 8 (Confidentiality), Article 11 (Indemnification), Article 13 (Dispute Resolution) and Article 14 and each of their subparts.
| 13. | Dispute Resolution. |
(a) Discussion by Senior Executives. If there is an unresolved matter, dispute or issue arising out of or relating to the interpretation, breach, performance or application of this Agreement for which neither Party has the final decision making authority as expressly provided elsewhere in this Agreement, either Party may refer such matter, dispute or issue to the Chief Executive Officer of Capricor and the President of JBI or their designee(s), in writing for further discussion and resolution. These individuals shall as soon as practicable meet and attempt in good faith to resolve the matter, dispute or issue and reach Agreement. These individuals may obtain the advice of other employees or consultants as they deem necessary or advisable in order to make the decision. If these individuals cannot reach Agreement as to the matter, dispute or issue within thirty (30) Calendar Days of the matter, dispute or issue being referred to them, then such matter, dispute or issue (an “Unresolved Issue”) will be resolved as provided in Section 13(b) (Arbitration) if the issue is material breach, and otherwise the issue will be resolved according to the position of the Party having the right and license under this Agreement or otherwise to act or proceed.
(b) Arbitration.
(i) If the Parties fail to resolve the Unresolved Issue which concerns the breach of an obligation or duty under this Agreement in mediation pursuant to Section 13(b) (Mediation), and a Party desires to pursue resolution of the Unresolved Issue, the Unresolved Issue shall be submitted by either party for resolution in arbitration pursuant to the then current CPR Non-Administered Arbitration Rules ("CPR Rules") (www.cpradr.org), except where they conflict with these provisions, in which case these provisions control. The arbitration will be held in New York, New York. All aspects of the arbitration shall be treated as confidential.
(ii) The arbitrators will be chosen from the CPR Panel of Distinguished Neutrals, unless a candidate not on such panel is approved by both parties. Each arbitrator shall be a lawyer with at least 15 years of experience with at least one company in the business of developing and marketing pharmaceutical products to treat, ameliorate, or cure human diseases or conditions whether in a corporate law department or otherwise, where such company has yearly reported sales of at least five hundred million US dollars ($500MM). To the extent that the Unresolved Issue requires special expertise, the parties will so inform CPR prior to the beginning of the selection process.
| CONFIDENTIAL | Page 23 |
(iii) The arbitration tribunal shall consist of three (3) arbitrators, of whom each party shall designate one in accordance with the "screened" appointment procedure provided in CPR Rule 5.4. The chair will be chosen in accordance with CPR Rule 6.4. A single arbitrator may be chosen in accordance with the CPR Rules if (a) the Unresolved Issue does not involve a determination of the existence of an uncured material breach, or (b) the aggregate award sought by the Parties is less than five million U.S. dollars ($5,000,000.00 US) and equitable relief is not sought. Candidates for the arbitrator position(s) may be interviewed by representatives of the Parties in advance of their selection, provided that all parties are represented.
(iv) The Parties agree to select the arbitrator(s) within thirty (30) days of initiation of the arbitration. The hearing will be concluded within six (6) months after selection of the arbitrator(s) and the award will be rendered within sixty (60) days of the conclusion of the hearing, or of any post-hearing briefing, which briefing will be completed by both sides within forty-five (45) days after the conclusion of the hearing. In the event the Parties cannot agree upon a schedule, then the arbitrator(s) shall set the schedule following the time limits set forth above as closely as practical.
(v) The hearing will be concluded in ten (10) hearing days or less. Multiple hearing days will be scheduled consecutively to the greatest extent possible. A transcript of the testimony adduced at the hearing shall be made and shall be made available to each party.
(vi) The arbitrator(s) shall be guided, but not bound, by the CPR Protocol on Disclosure of Documents and Presentation of Witnesses in Commercial Arbitration (www.cpradr.org) (the "Protocol"). The Parties will attempt to agree on modes of document disclosure, electronic discovery, witness presentation, etc. within the parameters of the Protocol. If the Parties cannot agree on discovery and presentation issues, the arbitrator(s) shall decide on presentation modes and provide for discovery within the Protocol, understanding that the Parties contemplate reasonable discovery.
(vii) The arbitrator(s) shall decide the merits of any Unresolved Issue in accordance with the law governing this Agreement, without application of any principle of conflict of laws that would result in reference to a different law. The arbitrator(s) may not apply principles such as "amiable compositeur" or "natural justice and equity."
(viii) The arbitrator(s) are expressly empowered to decide dispositive motions in advance of any hearing and shall endeavor to decide such motions as would a United States District Court Judge sitting in the jurisdiction whose substantive law governs.
| CONFIDENTIAL | Page 24 |
(ix) The arbitrator(s) shall render a written opinion stating the reasons upon which the award is based. The Parties consent to the jurisdiction of the United States District Court for the district in which the arbitration is held for the enforcement of these provisions and the entry of judgment on any award rendered hereunder. Should such court for any reason lack jurisdiction, any court with jurisdiction may act in the same fashion.
(x) Each Party has the right to seek from the appropriate court provisional remedies such as attachment, preliminary injunction, replevin, etc. to avoid irreparable harm, maintain the status quo, or preserve the subject matter of the Dispute. Rule 14 of the CPR Rules does not apply to this Agreement.
(xi) EACH PARTY HERETO WAIVES: (1) ITS RIGHT TO TRIAL OF ANY ISSUE BY JURY, (2) WITH THE EXCEPTION OF RELIEF MANDATED BY STATUTE, ANY CLAIM TO PUNITIVE, EXEMPLARY, MULTIPLIED, INDIRECT, CONSEQUENTIAL OR LOST PROFITS/REVENUES DAMAGES, AND (3) ANY CLAIM FOR ATTORNEY FEES COSTS AND PREJUDGMENT INTEREST.
| 14. | Miscellaneous |
14.1. Affiliates and Designees. The Parties shall have the right to exercise their respective rights, perform their respective obligations and/or receive performance of the other Party’s obligations hereunder through its Affiliates, designees, sublicensees or licensees.
14.2. Assignment. Neither this Agreement nor any rights or obligations hereunder may be assigned or otherwise transferred (whether by operation of Law, general succession or otherwise) by either Party without the prior written consent of the other Party, except that (a) either Party, upon prior written notice, may assign this Agreement to an Affiliate of such Party and (b) Capricor or any Affiliate may assign this Agreement in connection with the sale of all or substantially all of its assets or business to which this Agreement relates or pursuant to a Change of Control. Any assignment not in accordance with this Agreement will be void. Subject to the foregoing, the rights and obligations of the Parties under this Agreement will be binding upon and inure to the benefit of the successors and permitted assigns of the Parties. The acquiring entity of Capricor will agree in writing to assume all of Capricor’s obligations hereunder.
14.3. Choice of Law. This Agreement will be governed by, and enforced and construed in accordance with, the laws of the State of Delaware without regard to its conflicts of law provisions.
14.4. Construction. The definitions of the terms herein will apply equally to the singular and plural forms of the terms defined. Whenever the context may require, any pronoun will include the corresponding masculine, feminine and neuter forms. The words “include”, “includes” and “including” will be deemed to be followed by the phrase “without limitation.” The word “will” will be construed to have the same meaning and effect as the
| CONFIDENTIAL | Page 25 |
word “shall.” The Parties each acknowledge that they have had the advice of counsel with respect to this Agreement, that this Agreement has been jointly drafted, and that no rule of strict construction will be applied in the interpretation hereof. Unless the context requires otherwise, (a) any definition of or reference to any agreement, instrument or other document herein will be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein or therein), (b) any reference to any Laws herein will be construed as referring to such Laws as from time to time enacted, repealed or amended, (c) any reference herein to any person will be construed to include the person’s permitted successors and assigns, (d) the words “herein”, “hereof” and “hereunder”, and words of similar import, will be construed to refer to this Agreement in its entirety and not to any particular provision hereof, and (e) all references herein to Articles, Sections or Schedules, unless otherwise specifically provided, will be construed to refer to Articles, Sections or Schedules of this Agreement. This Agreement has been executed in English, and the English version of this Agreement will control.
14.5. Counterparts. This Agreement may be executed in counterparts with the same effect as if both Parties had signed the same document. All such counterparts will be deemed an original, will be construed together and will constitute one and the same instrument. Signature pages of this Agreement may be exchanged by facsimile or other electronic means without affecting the validity thereof.
14.6. Entire Agreement. This Agreement, including the attached Schedules constitutes the entire agreement between the Parties as to the subject matter of this Agreement, and supersedes and merges all prior discussions, representations, agreements and understandings regarding the same, except for the Mutual NDA executed by the parties on […***…] as amended on […***…].
14.7. Force Majeure. Neither Party will be liable for delay or failure in the performance of any of its obligations hereunder (other than the payment of money) if such delay or failure is due to causes beyond its reasonable control, including acts of God, fires, floods, earthquakes, labor strikes, acts of war, terrorism or civil unrest (“Force Majeure”); provided, however, that the affected Party notifies the other Party in writing within thirty (30) days of the Force Majeure event (and continues to provide monthly status updates to the other Party for the duration of the effect); further provided that the affected Party will use its reasonable efforts to avoid or remove such causes of non-performance and to mitigate the effect of such occurrence, and will continue performance with reasonable dispatch whenever such causes are removed.
14.8. Further Assurances. Each Party agrees to do and perform all such further acts and things and will execute and deliver such other agreements, certificates, instruments and documents necessary or that the other Party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and to evidence, perfect or otherwise confirm its rights hereunder.
*Confidential Treatment Requested
| CONFIDENTIAL | Page 26 |
14.9. Headings. Headings and captions are for convenience only and are not to be used in the interpretation of this Agreement.
14.10. Notices. Any notice required or permitted to be given by this Agreement will be in writing, in English, and will be delivered by hand or overnight courier with tracking capabilities addressed as set forth below unless changed by notice so given:
| If to Capricor: | Capricor, Inc. | |
| 8840 Wilshire Blvd., 2nd Floor |
| Beverly Hills, CA 90211 |
Attention:
[…***…]
Telephone: […***…]
Facsimile: […***…]
(with a copy to)
[…***…]
General Counsel
[…***…]
If to Licensee: Janssen Biotech, Inc.
Attention: President
[…***…]
(with a copy to) Johnson & Johnson
| One Johnson & Johnson Plaza |
| New Brunswick, NJ 08933 |
Attention: Chief Intellectual Property Officer
Facsimile: 732-524-5575
Any such notice will be deemed given on the date delivered. A Party may add, delete (so long as at least one person is remaining), or change the person or address to which notices should be sent at any time upon written notice delivered to the other Party in accordance with this Section 14.10 (Notices).
14.11. Relationship of the Parties. Each Party is an independent contractor under this Agreement. Nothing contained herein is intended or is to be construed so as to constitute Licensee and Capricor as partners, agents or joint venturers. Neither Party has any express or implied right or authority to assume or create any obligations on behalf of or in the name of the other Party or to bind the other Party to any contract, agreement or undertaking with any Third Party.
14.12. Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable, the provision will be considered severed from this Agreement and will not serve to invalidate any remaining provisions hereof. The Parties will negotiate in good faith to replace any invalid or unenforceable provision with a valid and enforceable one
*Confidential Treatment Requested
| CONFIDENTIAL | Page 27 |
such that the objectives contemplated by the Parties when entering this Agreement may be realized.
14.13. Third Party Beneficiaries. Except as expressly provided with respect to Capricor Indemnitees or Licensee Indemnitees in Article 11 (Indemnification), there are no third party beneficiaries intended hereunder and no Third Party will have any right or obligation hereunder.
14.14. Waivers and Modifications. The failure of any Party to insist on the performance of any obligation hereunder will not be deemed to be a waiver of such obligation. Waiver of any breach of any provision hereof will not be deemed to be a waiver of any other breach of such provision or any other provision on such occasion or any other occasion. No waiver, modification, release or amendment of any right or obligation under or provision of this Agreement will be valid or effective unless in writing and signed by all Parties hereto.
*********
(Signature page follows)
| CONFIDENTIAL | Page 28 |
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
| JANSSEN BIOTECH, INC. | CAPRICOR, INC. | |||
| By: | /s/ John Haney | By: | /s/ Linda Marbán | |
| Name: | John Haney | Name: | Linda Marbán | |
| Title: | Vice President - Immunology Marketing | Title: | Chief Executive Officer | |
[…***…]
*Confidential Treatment Requested
Capricor Third Party License Schedule (Schedule B)
License Agreement between Universita Degli Studi Di Roma “La Sapienza” effective June 21, 2006 (the “Rome License”),
Exclusive License Agreement between The Johns Hopkins University and Capricor, Inc., effective June 22, 2006 (the "Johns Hopkins License”), and
Exclusive License Agreement between Cedars-Sinai Medical Center and Capricor, Inc., effective January 4, 2010 as amended by Amendment to License Agreement dated February 25, 2013 and Second Amendment to License Agreement dated April 19, 2013 (the “Cedars-Sinai License”).
Johnson & Johnson Universal Financial Calendar Schedule

Exclusive License Agreement Term Sheet Schedule
[…***…]
*Confidential Treatment Requested
Press Release Schedule

Final- Execution Version
Capricor Therapeutics and Janssen Biotech, Inc. Enter into Exclusive License Option and Collaboration Agreement
LOS ANGELES, January , 2014 – Capricor Therapeutics, Inc. (OTCBB: CAPR) today announced that it has executed an exclusive license option and collaboration agreement with Janssen Biotech, Inc. (Janssen) for its cell therapy program for cardiovascular applications, including lead product, CAP-1002. CAP-1002 is an allogeneic cardiosphere derived cell (CDC) therapeutic under evaluation in patients who have suffered a large myocardial infarction. Pursuant to the agreement, Capricor and Janssen will collaborate on elements of cell manufacturing development . Capricor will contribute to the costs of the manufacturing collaboration, and will receive an upfront payment of
$12.5 million from Janssen.
Under the terms of the agreement, Janssen will have the right to enter into an exclusive license agreement for CAP-1002 following the review of six month data from the ongoing ALLSTAR trial , which is Capricor’s Phase I/II trial of CAP -1002. If Janssen exercises its option rights, Capricor will be eligible to receive up to $325 million in additional payments. In addition, a royalty would be paid on commercial sales of CAP-1002.
“This collaboration with Janssen, one of the world’s largest and most respected healthcare companies with a strong presence in cardiovascular and metabolism, is a tremendous milestone for Capricor Therapeutics, and an important validation of our lead product, CAP -1002 and the underlying science. . We are proud to be working with them to support the continued development CAP-1002, and for the additional non dilutive capital to further develop our research and development that will add to our pipeline said Capricor CEO, Dr. Linda Marbán.
About Capricor Therapeutics
Capricor Therapeutics, Inc. (CAPR), a publicly traded biotechnology company, is focused on the development of novel therapeutics to prevent and treat heart disease. The Company has two leading product candidates: CAP-1002 and Cenderitide. The Company was formed through the November 2013 merger between Capricor, Inc., a privately held company whose mission is to improve the treatment of heart disease by commercializing cardiac stem cell therapies for patients, and Nile Therapeutics, Inc., a clinical-stage biopharmaceutical company developing innovative products for the treatment of cardiovascular diseases. Capricor Therapeutics’ stock will begin trading under the symbol “CAPR” starting December 20, 2013. For additional information visit www.capricor.com.
About CAP-1002
CAP-1002, Capricor’s lead product candidate, is a proprietary allogeneic adult stem cell therapy for
the treatment of heart disease. The product is derived from donor heart tissue. The cells are expanded in the laboratory using a specialized process and then introduced directly into a patient’s heart via infusion into a coronary artery using standard cardiac catheterization techniques.
CAP-1002 is currently not an approved product and is strictly for investigational purposes.
Cautionary Note Regarding Forward-Looking Statements
Statements in this press release regarding the efficacy, safety, and intended utilization of Capricor’s product candidates; the conduct, size, timing and results of discovery efforts and clinical trials; plans regarding regulatory filings, future research and clinical trials; plans regarding current and future collaborative activities and the ownership of commercial rights; future royalty streams, and any other statements about Capricor’s management team’s future expectations, beliefs, goals, plans or prospects constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements that are not statements of historical fact (including statements containing the words "believes," "plans," "could," "anticipates," "expects," "estimates," "plans," "should," "target," "will," "would" and similar expressions) should also be considered to be forward-looking statements. There are a number of important factors that could cause actual results or events to differ materially from those indicated by such forward-looking statements.
More information about these and other risks that may impact our business are set forth in our Form 10-K for the year ended December 31, 2012, as filed with the Securities and Exchange Commission on June 21, 2013, in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2013, as filed with the Securities and Exchange Commission on November 14, 2013, and in our Definitive Proxy Statement on Schedule 14A, as filed with the Securities and Exchange Commission on October 10, 2013. All forward-looking statements in this press release are based on information available to us as of the date hereof, and we assume no obligation to update these forward-looking statements.
Six Month Top Line Report Schedule
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
Definitions
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested
Exclusive License Agreement Term Sheet
[…***…]
*Confidential Treatment Requested