| HOPE - 2 Open Label Extension (1 - Year Data Results) Trial conducted by Capricor National PI: Craig McDonald, M.D. (UC Davis) NASDAQ:CAPR |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Forward Looking Statements Statementsinthispresentationregardingtheefficacy,safety,andintendedutilizationofCapricor'sproductcandidates;theinitiation,conduct,size,timingandresultsofdiscoveryeffortsandclinicaltrials;thepaceofenrollmentofclinicaltrials;plansregardingregulatoryfilings,futureresearchandclinicaltrials;regulatorydevelopmentsinvolvingproducts,includingtheabilitytoobtainregulatoryapprovalsorotherwisebringproductstomarket;theabilitytoachieveproductmilestonesandtoreceivemilestonepaymentsfromcommercialpartners;plansregardingcurrentandfuturecollaborativeactivitiesandtheownershipofcommercialrights;scope,duration,validityandenforceabilityofintellectualpropertyrights;futureroyaltystreams,revenueprojections;expectationswithrespecttotheexpecteduseofproceedsfromtherecentlycompletedofferingsandtheanticipatedeffectsoftheofferings,andanyotherstatementsaboutCapricor'smanagementteam'sfutureexpectations,beliefs,goals,plansorprospectsconstituteforward-lookingstatementswithinthemeaningofthePrivateSecuritiesLitigationReformActof1995.Anystatementsthatarenotstatementsofhistoricalfact(includingstatementscontainingthewords"believes,""plans,""could,""anticipates,""expects,""estimates,""should,""target,""will,""would"andsimilarexpressions)shouldalsobeconsideredtobeforward-lookingstatements.Thereareanumberofimportantfactorsthatcouldcauseactualresultsoreventstodiffermateriallyfromthoseindicatedbysuchforward-lookingstatements.MoreinformationabouttheseandotherrisksthatmayimpactCapricor'sbusinessissetforthinCapricor'sAnnualReportonForm10-KfortheyearendedDecember31,2021asfiledwiththeSecuritiesandExchangeCommissiononMarch11,2022andinourQuarterlyReportonForm10-QforthequarterendedMarch31,2022asfiledwiththeSecuritiesandExchangeCommissiononMay11,2022.Allforward-lookingstatementsinthispressreleasearebasedoninformationavailabletoCapricorasofthedatehereof,andCapricorassumesnoobligationtoupdatetheseforward-lookingstatements.CAP-1002isanInvestigationalNewDrugandisnotapprovedforanyindications.NoneofCapricor’sexosome-basedcandidateshavebeenapprovedforclinicalinvestigation. 2 |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Call Participants •Linda Marban, Ph.D. –Chief Executive Officer, Capricor Therapeutics, Inc.•Dan Paulson, M.D. –Vice President of Clinical Development, Capricor Therapeutics, Inc.•AJ Bergmann, M.B.A. –Chief Financial Officer, Capricor Therapeutics, Inc. •Craig McDonald, M.D. –Professor and Chair of the Department of Physical Medicine and Rehabilitation and Director of the Neuromuscular Disease Clinics at the University of California, Davis. Dr. McDonald is an internationally recognized expert in the clinical management and rehabilitation of neuromuscular diseases including DMD. He is the national PI of Capricor’s HOPE-2 and HOPE-3 trials. •Suzanne Hendrix, Ph.D. –CEO Pentara Statistical Group, Dr. Hendrix has been instrumental in analysis and reporting for multiple regulatory submissions and authored or co-authored over 150 peer-reviewed publications related to both clinical trial results and statistical approaches for clinical trials, most of which relate to analysis and design of trials for neurodegenerative diseases. 3 |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. CAP-1002 Cell Therapy Does not act by «stemness»the cells do not engraft into host tissueSourced fromtransplant qualifiedheartsCAP-1002: Mechanism of action Cells secrete exosomes•Contain miRNAs, non-coding RNAs and proteins•Trigger natural signaling with target cells•Activate changes in cellular behaviorHas been investigated ineightclinical trialsClinical data demonstrating skeletel and cardiac improvements in DMD CAP-1002: Allogeneic Cardiosphere-Derived Cells (CDCs) 4 |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. CAP-1002 Infusion Protocol is Easy •I.V. (intravenous) administration every 3 months –~45 minutes Procedure•Simple oral premedication regimen before infusion •Safety profile: no treatment related SAEs reported through 94 infusions in ongoing HOPE-2 open label extension•CAP-1002 has been administered to over 200 subjects to date across multiple clinical trials5 |
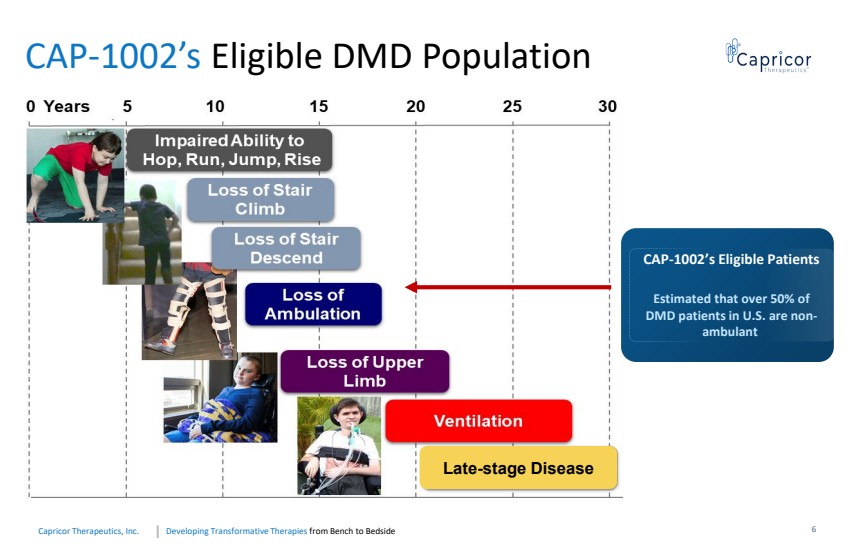
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. CAP-1002’s Eligible DMD Population CAP-1002’s EligiblePatientsEstimated that over 50% of DMD patients in U.S. are non-ambulant Late-stage Disease6 |
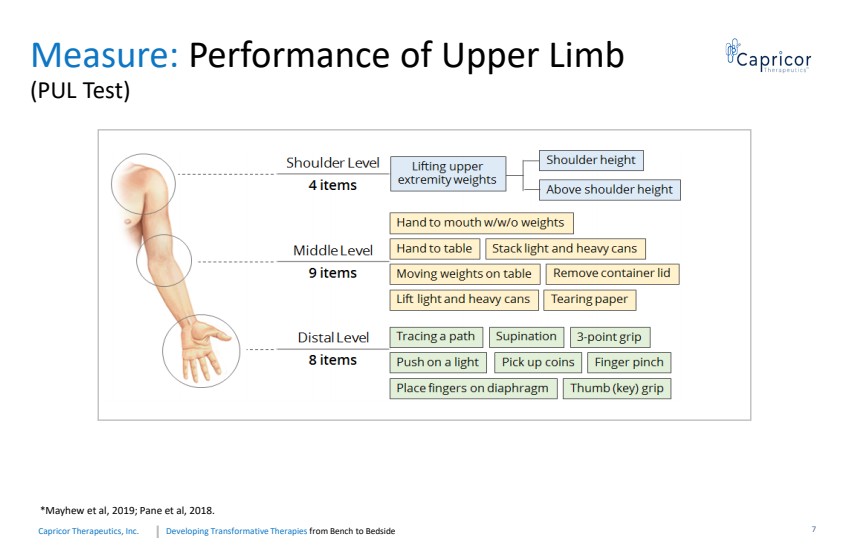
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Measure: Performance of Upper Limb (PUL Test) *Mayhew et al, 2019; Pane et al, 2018. 7 |
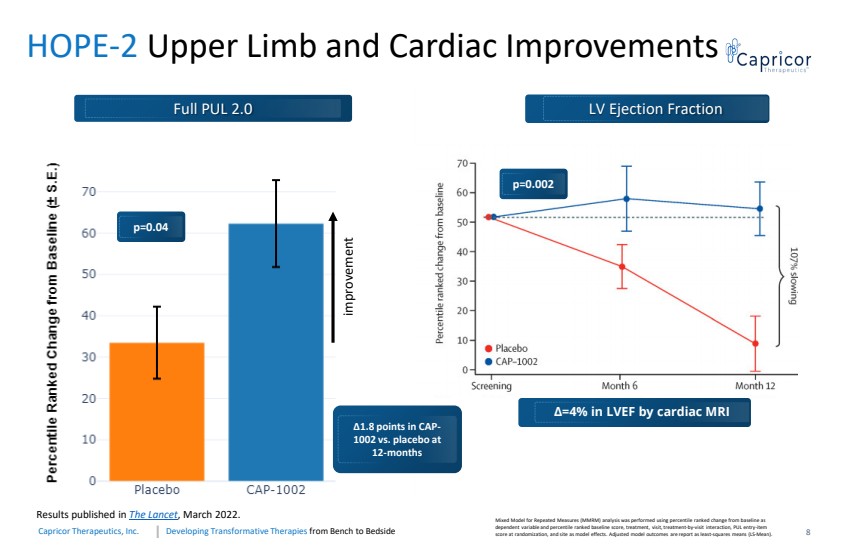
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. 8HOPE-2 Upper Limb and Cardiac Improvements Mixed Model for Repeated Measures (MMRM) analysis was performed using percentile ranked change from baseline as dependent variable and percentile ranked baseline score, treatment, visit, treatment-by-visit interaction, PUL entry-item score at randomization, and site as model effects. Adjusted model outcomes are report as least-squares means (LS-Mean). Δ1.8 points in CAP-1002 vs. placebo at 12-monthsimprovement p=0.04 Full PUL 2.0 Results published in The Lancet , March 2022. Δ=4% in LVEF by cardiac MRI LV Ejection Fraction p=0.002 |
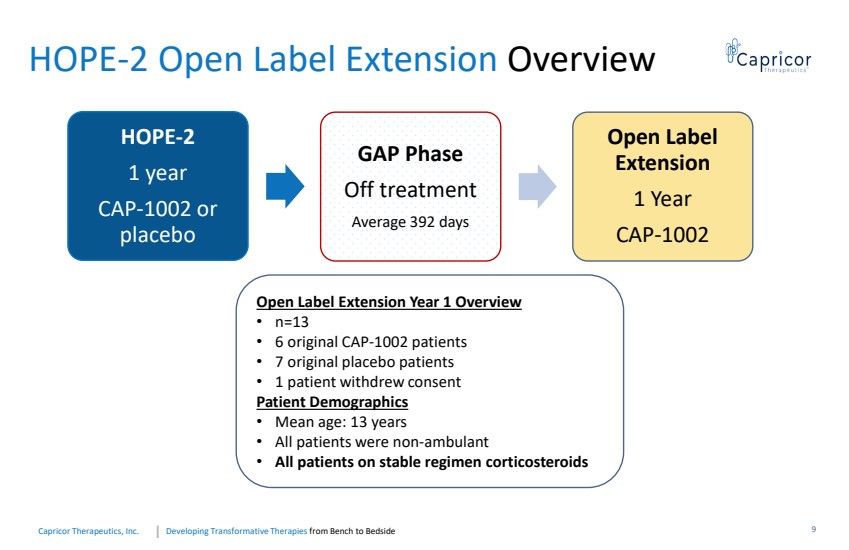
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. HOPE-2 Open Label Extension Overview Open Label Extension Year 1 Overview •n=13•6 original CAP-1002 patients•7 original placebo patients•1 patient withdrew consent Patient Demographics •Mean age: 13 years•All patients were non-ambulant •All patients on stable regimen corticosteroids HOPE-21 yearCAP-1002 or placebo GAP PhaseOff treatmentAverage 392 days Open Label Extension 1 Year CAP-10029 |
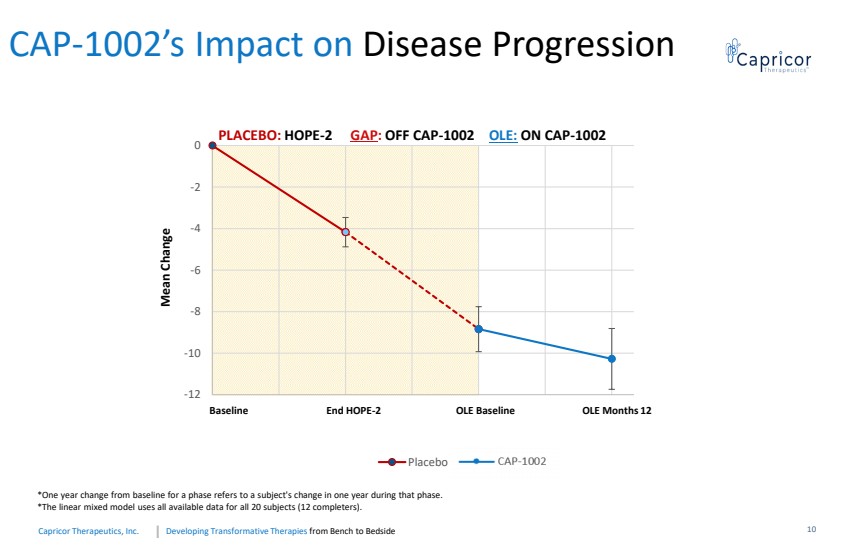
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. -12-10-8-6 -4 -20061218243036 Placebo Baseline End HOPE-2 OLE Baseline OLE Months 12Mean Change PLACEBO: HOPE-2GAP : OFFCAP-1002OLE: ON CAP-1002*Oneyearchangefrombaselineforaphasereferstoasubject'schangeinoneyearduringthatphase.*Thelinearmixedmodelusesallavailabledataforall20subjects(12completers). CAP-1002’s Impact on Disease Progression 10 |
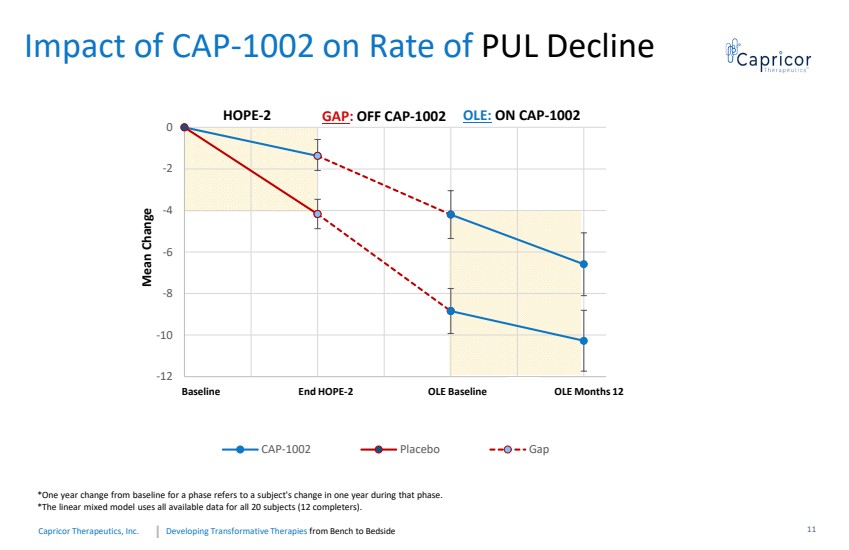
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. -12-10-8 -6-4-20061218243036 CAP-1002 Placebo Gap Baseline End HOPE-2 OLE Baseline OLE Months 12Mean Change HOPE-2GAP : OFF CAP-1002OLE: ON CAP-1002Impact of CAP-1002 on Rate of PUL Decline*Oneyearchangefrombaselineforaphasereferstoasubject'schangeinoneyearduringthatphase.*Thelinearmixedmodelusesallavailabledataforall20subjects(12completers).11 |
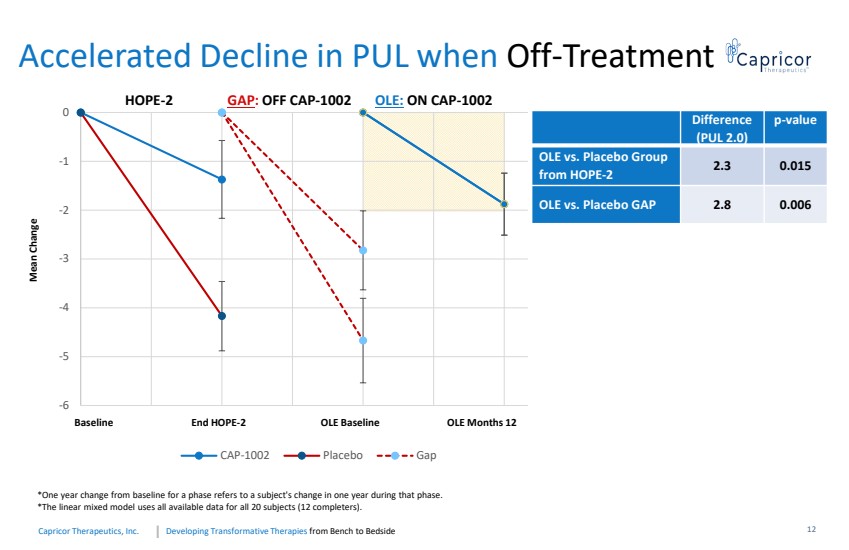
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Accelerated Decline in PUL when Off-TreatmentMean ChangeHOPE-2GAP : OFF CAP-1002OLE: ON CAP-1002 Difference (PUL 2.0)p-valueOLE vs. Placebo Group from HOPE-22.30.015OLE vs. Placebo GAP2.80.006*Oneyearchangefrombaselineforaphasereferstoasubject'schangeinoneyearduringthatphase.*Thelinearmixedmodelusesallavailabledataforall20subjects(12completers). -6-5-4-3 -2-1 0 CAP-1002 Placebo Gap Baseline End HOPE-2 OLE Baseline OLE Months 1212 |
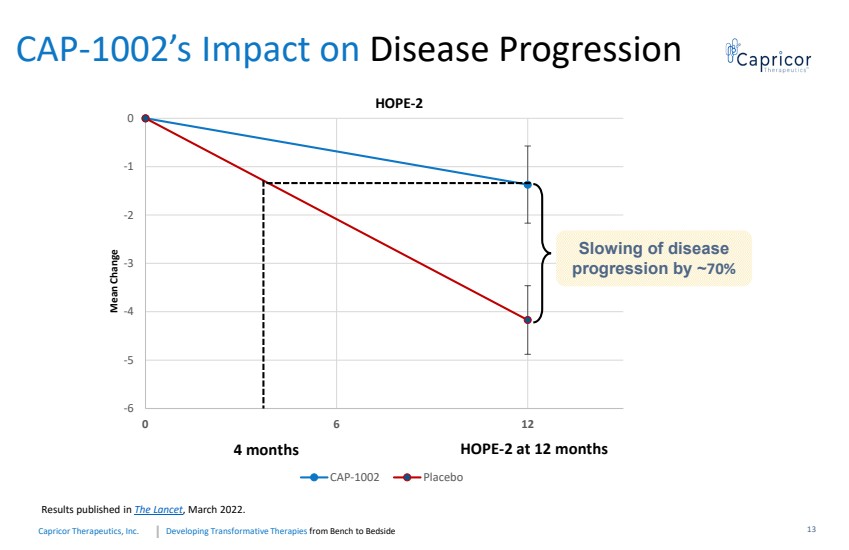
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. CAP-1002’s Impact on Disease Progression -6-5-4-3 -2-100612 CAP-1002 Placebo HOPE-2 at 12 months4 monthsMean ChangeResults published in The Lancet , March 2022. Slowing of disease progression by ~70%HOPE-213 |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Time is Muscle for DMD Patients•CAP-1002: Potential Disease Modifying Benefit•Patients on therapy experience a slower progression of their disease•Urgency to initiate therapy: while treatment benefits are maintained (compared to placebo patients), loss of PUL points are never recovered•Potential sustained benefit: durable benefit of treatment at 2 years•Safety profile of CAP-1002 reinforced•Preserved cardiac ejection fraction shown in HOPE-2•HOPE-3 pivotal Phase 3 clinical trial open for enrollment •Primary efficacy endpoint: PUL 2.0 at 12 months14 |
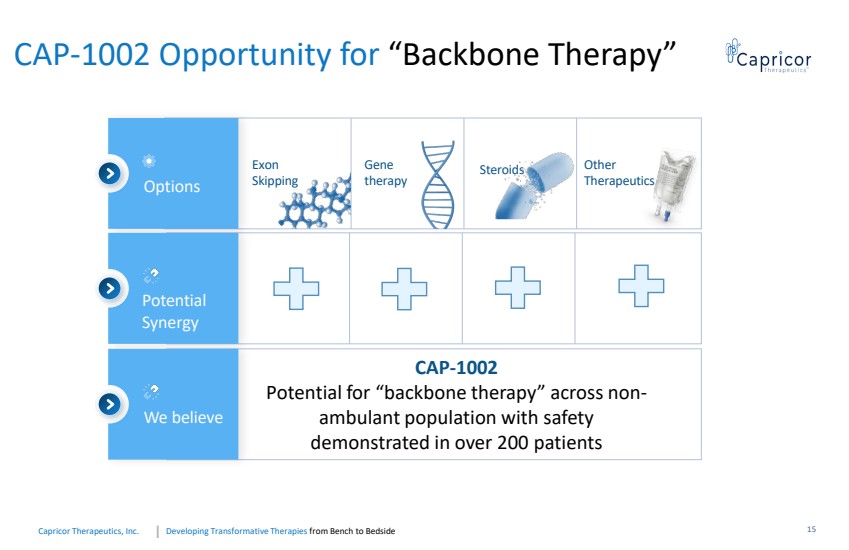
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. CAP-1002 Opportunity for “Backbone Therapy” Options We believe Exon SkippingGene therapySteroidsCAP-1002Potential for “backbone therapy” across non-ambulant population with safety demonstrated in over 200 patients Potential Synergy Other Therapeutics 15 |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Acknowledgments 16 Patients and their families who participated in the HOPE-2 and HOPE-2 OLE Studies•Parent Project Muscular Dystrophy•Craig McDonald, M.D. (UC Davis)•Coalition Duchenne•CureDuchenne•The Jett Foundation•Action Duchenne•MDA•Casimir•NS Pharma •Capricor’s DMD Advisory Board•Cuixia Tian, M.D. (CCHMC)•Russell Butterfield, M.D. (University of Utah)•Richard Finkel, M.D. (Nemours Children’s Hospital)•Joanne Janas, M.D. (Children’s Hospital of Colorado)•Matthew Harmelink, M.D. (Children’s Hospital of Wisconsin)•Arun Varadhachary, M.D. (Washington University, Saint Louis Children’s Hospital)•Brenda Wong, M.D. (University of Massachusetts)•Katherine Mathews, M.D.(University of Iowa, Children’s Hospital) |
| Developing Transformative Therapiesfrom Bench to BedsideCapricorTherapeutics, Inc. Q&A17 |